Magnetic systems for cancer immunotherapy
- PMID: 34522583
- PMCID: PMC8424374
- DOI: 10.1016/j.apsb.2021.03.023
Magnetic systems for cancer immunotherapy
Abstract
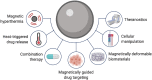
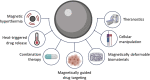
Immunotherapy is a rapidly developing area of cancer treatment due to its higher specificity and potential for greater efficacy than traditional therapies. Immune cell modulation through the administration of drugs, proteins, and cells can enhance antitumoral responses through pathways that may be otherwise inhibited in the presence of immunosuppressive tumors. Magnetic systems offer several advantages for improving the performance of immunotherapies, including increased spatiotemporal control over transport, release, and dosing of immunomodulatory drugs within the body, resulting in reduced off-target effects and improved efficacy. Compared to alternative methods for stimulating drug release such as light and pH, magnetic systems enable several distinct methods for programming immune responses. First, we discuss how magnetic hyperthermia can stimulate immune cells and trigger thermoresponsive drug release. Second, we summarize how magnetically targeted delivery of drug carriers can increase the accumulation of drugs in target sites. Third, we review how biomaterials can undergo magnetically driven structural changes to enable remote release of encapsulated drugs. Fourth, we describe the use of magnetic particles for targeted interactions with cellular receptors for promoting antitumor activity. Finally, we discuss translational considerations of these systems, such as toxicity, clinical compatibility, and future opportunities for improving cancer treatment.
Keywords: BW, body weight; Biomaterials; CpG, cytosine-phosphate-guanine; DAMP, damage associated molecular pattern; Drug delivery; EPR, enhanced permeability and retention; FFR, field free region; HS-TEX, heat-stressed tumor cell exosomes; HSP, heat shock protein; ICD, immunogenic cell death; IVIS, in vivo imaging system; Immunotherapy; MICA, MHC class I-related chain A; MPI, magnetic particle imaging; Magnetic hyperthermia; Magnetic nanoparticles; Microrobotics; ODNs, oligodeoxynucleotides; PARP, poly(adenosine diphosphate-ribose) polymerase; PDMS, polydimethylsiloxane; PEG, polyethylene glycol; PLGA, poly(lactic-co-glycolic acid); PNIPAM, poly(N-isopropylacrylamide); PVA, poly(vinyl alcohol); SDF, stromal cell derived-factor; SID, small implantable device; SLP, specific loss power.
© 2021 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures






References
-
- Reck M., Rodríguez-Abreu D., Robinson A.G., Hui R., Csőszi T., Fülöp A. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. - PubMed
-
- Sarfaty M., Hall P.S., Chan K.K., Virik K., Leshno M., Gordon N. Cost-effectiveness of pembrolizumab in second-line advanced bladder cancer. Eur Urol. 2018;74:57–62. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

