A review of existing strategies for designing long-acting parenteral formulations: Focus on underlying mechanisms, and future perspectives
- PMID: 34522592
- PMCID: PMC8424287
- DOI: 10.1016/j.apsb.2021.05.002
A review of existing strategies for designing long-acting parenteral formulations: Focus on underlying mechanisms, and future perspectives
Abstract
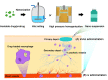
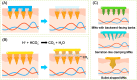
The need for long-term treatments of chronic diseases has motivated the widespread development of long-acting parenteral formulations (LAPFs) with the aim of improving drug pharmacokinetics and therapeutic efficacy. LAPFs have been proven to extend the half-life of therapeutics, as well as to improve patient adherence; consequently, this enhances the outcome of therapy positively. Over past decades, considerable progress has been made in designing effective LAPFs in both preclinical and clinical settings. Here we review the latest advances of LAPFs in preclinical and clinical stages, focusing on the strategies and underlying mechanisms for achieving long acting. Existing strategies are classified into manipulation of in vivo clearance and manipulation of drug release from delivery systems, respectively. And the current challenges and prospects of each strategy are discussed. In addition, we also briefly discuss the design principles of LAPFs and provide future perspectives of the rational design of more effective LAPFs for their further clinical translation.
Keywords: 2′-F, 2′-fluoro; 2′-O-MOE, 2′-O-(2-methoxyethyl); 2′-OMe, 2′-O-methyl; 3D, three-dimensional; ART, antiretroviral therapy; ASO, antisense oligonucleotide; Biomimetic strategies; Chemical modification; DDS, drug delivery systems; ECM, extracellular matrix; ENA, ethylene-bridged nucleic acid; ESC, enhanced stabilization chemistry; EVA, ethylene vinyl acetate; Fc/HSA fusion; FcRn, Fc receptor; GLP-1, glucagon like peptide-1; GS, glycine–serine; HA, hyaluronic acid; HES, hydroxy-ethyl-starch; HP, hypoparathyroidism; HSA, human serum albumin; Hydrogels; ISFI, in situ forming implants; IgG, immunoglobulin G; Implantable systems; LAFs, long-acting formulations; LAPFs, long-acting parenteral formulations; LNA, locked nucleic acid; Long-acting; MNs, microneedles; Microneedles; NDS, nanochannel delivery system; NPs, nanoparticles; Nanocrystal suspensions; OA, osteoarthritis; PCPP-SA, poly(1,3-bis(carboxyphenoxy)propane-co-sebacic-acid); PEG, polyethylene glycol; PM, platelet membrane; PMPC, poly(2-methyacryloyloxyethyl phosphorylcholine); PNAs, peptide nucleic acids; PS, phase separation; PSA, polysialic acid; PTH, parathyroid hormone; PVA, polyvinyl alcohol; RBCs, red blood cells; RES, reticuloendothelial system; RNAi, RNA interference; SAR, structure‒activity relationship; SCID, severe combined immunodeficiency; SE, solvent extraction; STC, standard template chemistry; TNFR2, tumor necrosis factor receptor 2; hGH, human growth hormone; im, intramuscular; iv, intravenous; mPEG, methoxypolyethylene glycol; sc, subcutaneous.
© 2021 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V.
Conflict of interest statement
The authors declare no conflicting interests in connection with this article.
Figures








References
-
- Lindenmayer J.P., Glick I.D., Talreja H., Underriner M. Persistent barriers to the use of long-acting injectable antipsychotics for the treatment of schizophrenia. J Clin Psychopharmacol. 2020;40:346–349. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

