Delivery strategies of amphotericin B for invasive fungal infections
- PMID: 34522599
- PMCID: PMC8424280
- DOI: 10.1016/j.apsb.2021.04.010
Delivery strategies of amphotericin B for invasive fungal infections
Abstract
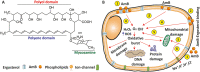
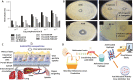
Invasive fungal infections (IFIs) represent a growing public concern for clinicians to manage in many medical settings, with substantial associated morbidities and mortalities. Among many current therapeutic options for the treatment of IFIs, amphotericin B (AmB) is the most frequently used drug. AmB is considered as a first-line drug in the clinic that has strong antifungal activity and less resistance. In this review, we summarized the most promising research efforts on nanocarriers for AmB delivery and highlighted their efficacy and safety for treating IFIs. We have also discussed the mechanism of actions of AmB, rationale for treating IFIs, and recent advances in formulating AmB for clinical use. Finally, this review discusses some practical considerations and provides recommendations for future studies in applying AmB for combating IFIs.
Keywords: ABCD, AmB colloidal dispersion; AIDS, acquired immunodeficiency syndrome; AP, antisolvent precipitation; ARDS, acute respiratory distress syndrome; AmB, amphotericin B; AmB-GCPQ, AmB-encapsulated N-palmitoyl-N-methyl-N,N-dimethyl-N,N,N-trimethyl-6-O-glycol-chitosan nanoparticles; AmB-IONP, AmB-loaded iron oxide nanoparticles; AmB-PM, AmB-polymeric micelles; AmB-SD, AmB sodium deoxycholate; AmBd, AmB deoxycholate; Amphotericin B; Aspergillus fumigatus, A. fumigatus; BBB, blood‒brain barrier; BCS, biopharmaceutics classification system; BDDE, butanediol diglycidyl ether; BSA, bovine serum albumin; BUN, blood urea nitrogen; C. Albicans, Candida Albicans; CFU, colony-forming unit; CLSM, confocal laser scanning microscope; CMC, carboxymethylated l-carrageenan; CP, chitosan-polyethylenimine; CS, chitosan; Conjugates; DDS, drug delivery systems; DMPC, dimyristoyl phosphatidyl choline; DMPG, dimyristoyl phosphatidylglycerole; DMSA, dimercaptosuccinic acid; Drug delivery; GNPs, gelatin nanoparticles; HPH, high-pressure homogenization; HPMC, hydroxypropyl methylcellulose; ICV, intensive care unit; IFIs, invasive fungal infections; Invasive fungal infections; L-AmB, liposomal AmB; LNA, linolenic acid; MAA, methacrylic acid; MFC, minimum fungicidal concentrations; MIC, minimum inhibitory concentration; MN, microneedles; MOP, microneedle ocular patch; MPEG-PCL, monomethoxy poly(ethylene glycol)-poly(epsilon-caprolactone); NEs, nanoemulsions; NLC, nanostructured lipid carriers; NPs, nanoparticles; Nanoparticles; P-407, poloxamer-407; PAM, polyacrylamide; PCL, polycaprolactone; PDA, poly(glycolic acid); PDLLA, poly(d,l-lactic acid); PDLLGA, poly(d,l-lactic-co-glycolic acid); PEG, poly(ethylene glycol); PEG-DSPE, PEG-lipid poly(ethylene glycol)-distearoylphosphatidylethanolamine; PEG-PBC, phenylboronic acid-functionalized polycarbonate/PEG; PEG-PUC, urea-functionalized polycarbonate/PEG; PGA-PPA, poly(l-lysine-b-l-phenylalanine) and poly(l-glutamic acid-b-l-phenylalanine); PLA, poly(lactic acid); PLGA, polyvinyl alcohol poly(lactic-co-glycolic acid); PLGA-PLH-PEG, PLGA-b-poly(l-histidine)-b-poly(ethylene glycol); PMMA, poly(methyl methacrylate); POR, porphyran; PVA, poly(vinyl alcohol); PVP, polyvinylpyrrolidone; Poor water-solubility; RBCs, red blood cells; RES, reticuloendothelial system; ROS, reactive oxygen species; SEM, scanning electron microscope; SL-AmB, sophorolipid-AmB; SLNs, solid lipid nanoparticles; Topical administration; Toxicity; γ-CD, γ-cyclodextrin; γ-PGA, γ-poly(gamma-glutamic acid.
© 2021 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V.
Conflict of interest statement
All the authors declare that this article content has no conflict of interest.
Figures






References
-
- Mellinghoff S.C., Panse J., Alakel N., Behre G., Buchheidt D., Christopeit M. Primary prophylaxis of invasive fungal infections in patients with haematological malignancies: 2017 update of the recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society for Haematology and Medical Oncology (DGHO) Ann Hematol. 2018;97:197–207. - PMC - PubMed
-
- Pappas P.G., Lionakis M.S., Arendrup M.C., Ostrosky-Zeichner L., Kullberg B.J. Invasive candidiasis. Nat Rev Dis Primers. 2018;4:18026. - PubMed
-
- Denning D.W., Kneale M., Sobel J.D., Rautemaa-Richardson R. Global burden of recurrent vulvovaginal candidiasis: a systematic review. Lancet Infect Dis. 2018;18:e339–e347. - PubMed
-
- Enoch D., Ludlam H., Brown N. Invasive fungal infections: a review of epidemiology and management options. J Med Microbiol. 2006;55:809–818. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

