Vaccination reduces need for emergency care in breakthrough COVID-19 infections: A multicenter cohort study
- PMID: 34522911
- PMCID: PMC8428472
- DOI: 10.1016/j.lana.2021.100065
Vaccination reduces need for emergency care in breakthrough COVID-19 infections: A multicenter cohort study
Abstract
Background: While recent literature has shown the efficacy of the SARS-CoV-2 vaccine in preventing infection, it's impact on need for emergency care/hospitalization in breakthrough infections remain unclear, particularly in regions with a high rate of variant viral strains. We aimed to determine if vaccination reduces hospital visits in breakthrough COVID-19.
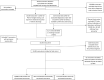
Methods: This observational cohort analysis compared unvaccinated (UV), partially vaccinated (PV), and fully vaccinated (FV) adult patients with SARS-CoV-2 infection requiring emergency care(EC)/hospitalization within an eight-hospital system in Michigan. Demographic and clinical variables were obtained from the electronic record. Vaccination data was obtained from the Michigan Care Improvement Registry and Centers for Disease Control vaccine tracker. Primary endpoint was rate of emergency care/hospitalization encounters among patients diagnosed with COVID-19. Secondary outcome was severe disease-composite outcome (ICU, mechanical ventilation, or in-hospital death).
Findings: Between December 15,2020 and April 30,2021, 11,834 EC encounters were included:10,880 (91.9%) UV, 825 (7%) PV, 129 (1.1%) FV. Average age was 53.0 ± 18.2 and 52.8% were female. Accounting for the SARS-CoV-2 vaccination population groups in Michigan, the ED encounters/hospitalizations rate relevant to COVID-19 was 96% lower in FV versus UV (multiplicative effect:0.04, 95% CI 0.03 to 0.06, p < 0.001) in negative binomial regression. COVID-19 EC visits rate peaked at 22.61, 12.88, and 1.29 visits per 100000 for the UV, PV, and FV groups, respectively. In the propensity-score matching weights analysis, FV had a lower risk of composite disease compared to UV but statistically insignificant (HR 0.84, 95% CI 0.52 to 1.38).
Interpretation: The need for emergency care/hospitalization due to breakthrough COVID-19 is an exceedingly rare event in fully vaccinated patients. As vaccination has increased regionally, EC visits amongst fully vaccinated individuals have remained low and occur much less frequently than unvaccinated individuals. If hospital-based treatment is required, elderly patients with significant comorbidities are at high-risk for severe outcomes regardless of vaccination status.
Keywords: COVID-19; SARS-CoV-2; coronavirus; intensive care unit; mechanical ventilation; mortality; vaccination; variants.
© 2021 The Author(s). Published by Elsevier Ltd.
Conflict of interest statement
None.
Figures

References
-
- Johns Hopkins University of Medicine . COVID-19 Map - Johns Hopkins Coronavirus Resource Center. 2020. https://coronavirus.jhu.edu/map.html Accessed October 15.
-
- Azar AM. Emergency Use Authorization Declaration. Published online 2020. https://www.federalregister.gov/documents/2020/04/01/2020-06905/emergenc...
-
- US Food and Drug Administration . FDA Issues Emergency Use Authorization for Third COVID-19 Vaccine. 2021. https://www.fda.gov/news-events/press-announcements/fda-issues-emergency... Accessed April 10.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

