Leptin as a key regulator of the adipose organ
- PMID: 34523036
- PMCID: PMC8873071
- DOI: 10.1007/s11154-021-09687-5
Leptin as a key regulator of the adipose organ
Abstract
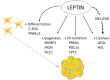
Leptin is a hormone primarily produced by the adipose tissue in proportion to the size of fat stores, with a primary function in the control of lipid reserves. Besides adipose tissue, leptin is also produced by other tissues, such as the stomach, placenta, and mammary gland. Altogether, leptin exerts a broad spectrum of short, medium, and long-term regulatory actions at the central and peripheral levels, including metabolic programming effects that condition the proper development and function of the adipose organ, which are relevant for its main role in energy homeostasis. Comprehending how leptin regulates adipose tissue may provide important clues to understand the pathophysiology of obesity and related diseases, such as type 2 diabetes, as well as its prevention and treatment. This review focuses on the physiological and long-lasting regulatory effects of leptin on adipose tissue, the mechanisms and pathways involved, its main outcomes on whole-body physiological homeostasis, and its consequences on chronic diseases.
Keywords: Adipose organ; Adiposity; Development; Energy homeostasis; Leptin; Metabolic programming.
© 2021. The Author(s).
Conflict of interest statement
No conflict of interest.
Figures




References
-
- Zhang Y, et al. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372(6505):425–432. - PubMed
-
- Palou A, et al. Obesity: molecular bases of a multifactorial problem. Eur J Nutr. 2000;39(4):127–144. - PubMed
-
- Friedman JM. Leptin and the endocrine control of energy balance. Nat Metab. 2019;1(8):754–764. - PubMed
-
- Cinti S. The adipose organ: endocrine aspects and insights from transgenic models. Eat Weight Disord. 2001;6(3 Suppl):4–8. - PubMed
-
- Campfield LA, et al. Recombinant mouse OB protein: evidence for a peripheral signal linking adiposity and central neural networks. Science. 1995;269(5223):546–549. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

