Lamotrigine in the maintenance treatment of bipolar disorder
- PMID: 34523118
- PMCID: PMC8440301
- DOI: 10.1002/14651858.CD013575.pub2
Lamotrigine in the maintenance treatment of bipolar disorder
Abstract
Background: Bipolar disorder is a chronic mental disorder with repetitive mania/hypomania as well as depressive episodes, which eventually results in marked impairment in overall functioning and health-related quality of life. A worldwide prevalence rate of 2.4% has been reported. The risk of suicide is higher in people with bipolar disorder than those with other mental disorders. Therefore, effective management of bipolar disorder in the maintenance period is warranted to minimize the risk of relapse or recurrence. Although lithium has been the standard treatment of bipolar disorder for many years, it is associated with adverse effects and teratogenicity. Lamotrigine is approved to be expected for prevention of recurrence for the maintenance treatment of bipolar disorder. In addition, lamotrigine is as effective as lithium. Therefore, we performed a systematic review to confirm the efficacy and safety of lamotrigine in the maintenance treatment of bipolar disorder.
Objectives: To assess the efficacy and tolerability of lamotrigine in the maintenance treatment of bipolar disorder.
Search methods: We searched Ovid MEDLINE, Embase, PsycINFO, the Cochrane Common Mental Disorders Group's Specialized Register (CCMDCTR) and the Cochrane Central Register of Controlled Trials (CENTRAL) from inception to 21 May 2021. We also searched international trial registries and contacted experts in the field.
Selection criteria: We included randomized controlled trials enrolling adults with bipolar disorder who were treated with lamotrigine, placebo or lithium.
Data collection and analysis: Two reviews authors independently checked the eligibility of studies and extracted data using a standardized form. Data extracted included study characteristics, participant characteristics, intervention details, settings, and outcome measures in the term of efficacy and tolerability. Study information were then entered into RevMan web.
Main results: We included 11 studies with a total of 2314 participants in this review; 1146 were randomized to lamotrigine, 869 were randomized to placebo and, 299 to lithium. We rated all studies as having an unclear risk of bias in at least one domain of Cochrane's tool for assessing risk of bias, with the most commonly observed weakness being selection bias (random sequence generation and allocation concealment). We judged five studies to be at a high risk of detection bias (blinding of outcome assessment). These potential biases pose as major threat to the validity of the included studies in this review. Outcomes of efficacy showed a possible advantage of lamotrigine over placebo. The estimated risk ratio (RR) for recurrence of manic symptom at one year as measured by the Young Mania Rating Scale (YMRS) was 0.67, (95% confidence interval (CI) 0.51 to 0.87; 3 studies, 663 participants; low-certainty evidence) in favor of lamotrigine. The RR of clinical worsening with the need for additional psychotropic treatment (RR 0.82, 95% CI 0.70 to 0.98; 4 studies, 756 participants) based on moderate-certainty evidence. The possible benefits of lamotrigine were also seen for the outcome of treatment withdrawal due to any reason at 6-12 months after treatment (RR 0.88, 95% CI 0.78 to 0.99; 4 studies, 700 participants; moderate-certainty evidence). Regarding tolerability, our analyses showed that the incidence rates of adverse effects were similar between the lamotrigine group and the placebo group (short-term effect: RR 1.07, 95% CI 0.81 to 1.42; 5 studies, 1138 participants; very low-certainty evidence; long-term effect: RR 0.97, 95% CI 0.77 to 1.23; 4 studies, 756 participants; moderate-certainty evidence). In the comparison between lamotrigine and lithium, efficacy was similar between groups except for recurrence of mania episode at one year. Recurrence of manic symptoms was higher in the lamotrigine group than that of the lithium group (RR 2.13, 95% CI 1.32 to 3.44; 3 studies, 602 participants; moderate-certainty evidence). Analysis of adverse effects at 6-12 months showed that a lower proportion of participants experienced at least one adverse effect when treated with lamotrigine compared to lithium (RR 0.70, 95% CI 0.51 to 0.96; 4 studies, 691 participants; moderate-certainty evidence).
Authors' conclusions: Low- to moderate-certainty evidence collectively suggests that lamotrigine may be superior to placebo as a treatment modality for bipolar disorder. In comparison to lithium, people with bipolar disorder seem to tolerate lamotrigine better in the long run; however, the demonstrated efficacy in the maintenance of bipolar disorder was similar between the two groups.
Trial registration: ClinicalTrials.gov NCT00274677 NCT00056277 NCT00550407 NCT00226135 NCT01588457.
Copyright © 2021 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
YH: none. KK: none. NW: none. TF: none. SS: none.
Figures
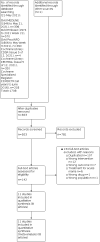

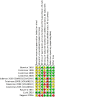

















Comment in
-
Lamotrigine in the Maintenance Treatment of Bipolar Disorder.Am Fam Physician. 2022 Sep;106(3):249-250. Am Fam Physician. 2022. PMID: 36126005 No abstract available.
Similar articles
-
Valproic acid, valproate and divalproex in the maintenance treatment of bipolar disorder.Cochrane Database Syst Rev. 2013 Oct 17;2013(10):CD003196. doi: 10.1002/14651858.CD003196.pub2. Cochrane Database Syst Rev. 2013. PMID: 24132760 Free PMC article.
-
Topical clonidine for neuropathic pain in adults.Cochrane Database Syst Rev. 2022 May 19;5(5):CD010967. doi: 10.1002/14651858.CD010967.pub3. Cochrane Database Syst Rev. 2022. PMID: 35587172 Free PMC article.
-
Pregabalin add-on for drug-resistant focal epilepsy.Cochrane Database Syst Rev. 2022 Mar 29;3(3):CD005612. doi: 10.1002/14651858.CD005612.pub5. Cochrane Database Syst Rev. 2022. PMID: 35349176 Free PMC article.
-
Electronic cigarettes for smoking cessation.Cochrane Database Syst Rev. 2022 Nov 17;11(11):CD010216. doi: 10.1002/14651858.CD010216.pub7. Cochrane Database Syst Rev. 2022. Update in: Cochrane Database Syst Rev. 2024 Jan 8;1:CD010216. doi: 10.1002/14651858.CD010216.pub8. PMID: 36384212 Free PMC article. Updated.
-
Electronic cigarettes for smoking cessation.Cochrane Database Syst Rev. 2021 Sep 14;9(9):CD010216. doi: 10.1002/14651858.CD010216.pub6. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 Nov 17;11:CD010216. doi: 10.1002/14651858.CD010216.pub7. PMID: 34519354 Free PMC article. Updated.
Cited by
-
Optimization of Therapy in Patients with Epilepsy and Psychiatric Comorbidities: Key Points.Curr Neuropharmacol. 2023;21(8):1755-1766. doi: 10.2174/1570159X20666220526144314. Curr Neuropharmacol. 2023. PMID: 35619263 Free PMC article.
-
Lamotrigine Therapy: Relation Between Treatment of Bipolar Affective Disorder and Incidence of Stevens-Johnson Syndrome-A Narrative Review of the Existing Literature.J Clin Med. 2025 Jun 10;14(12):4103. doi: 10.3390/jcm14124103. J Clin Med. 2025. PMID: 40565849 Free PMC article. Review.
-
Efficient Approaches to the Design of Six-Membered Polyazacyclic Compounds-Part 1: Aromatic Frameworks.Molecules. 2025 Aug 4;30(15):3264. doi: 10.3390/molecules30153264. Molecules. 2025. PMID: 40807439 Free PMC article. Review.
-
Effect of Lamotrigine on Refractory Epilepsy: Clinical Outcomes and EEG Changes.Int J Gen Med. 2025 Jan 21;18:281-290. doi: 10.2147/IJGM.S505040. eCollection 2025. Int J Gen Med. 2025. PMID: 39867247 Free PMC article.
-
Anti-seizure Medications: Challenges and Opportunities.CNS Neurol Disord Drug Targets. 2024;23(9):1120-1133. doi: 10.2174/0118715273275793231030060833. CNS Neurol Disord Drug Targets. 2024. PMID: 38192128 Review.
References
References to studies included in this review
Bowden 2003 {published data only}
-
- Bowden CL, Calabrese JR, Sachs G, Yatham LN, Asghar SA, Hompland M, et al, Lamictal 606 Study Group. A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently manic or hypomanic patients with bipolar I disorder. Archives of General Psychiatry 2003;60(4):392-400. - PubMed
Calabrese 1999 {published data only}
-
- Calabrese JR, Bowden CL, Sachs GS, Ascher JA, Monaghan E, Rudd GD, Lamictal 602 Study Group. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. Journal of Clinical Psychiatry 1999;60(2):79-88. - PubMed
-
- Calabrese JR, Huffman RF, White RL, Edwards S, Thompson TR, Ascher JA, et al. Lamotrigine in the acute treatment of bipolar depression: results of five double-blind, placebo-controlled clinical trials. Bipolar Disorders 2008;10(2):323-33. - PubMed
Calabrese 2000 {published data only}
-
- Calabrese JR, Suppes T, Bowden CL, Sachs GS, Swann AC, McElroy SL, et al, Lamictal 614 Study Group. A double-blind, placebo-controlled, prophylaxis study of lamotrigine in rapid-cycling bipolar disorder. Journal of Clinical Psychiatry 2000;61(11):841-50. - PubMed
Calabrese 2003 {published data only}
-
- Calabrese JR, Bowden CL, Sachs G, Yatham LN, Behnke K, Mehtonen OP, et al, Lamictal 605 Study Group. A placebo-controlled 18-month trial of lamotrigine and lithium maintenance treatment in recently depressed patients with bipolar I disorder. Journal of Clinical Psychiatry 2003;64(9):1013-24. - PubMed
Calabrese 2008 [GW603/SCAA2010] {published data only}
-
- Calabrese JR, Huffman RF, White RL, Edwards S, Thompson TR, Ascher JA, et al. Lamotrigine in the acute treatment of bipolar depression: results of five double-blind, placebo-controlled clinical trials. Bipolar Disorders 2008;10(2):323-33. - PubMed
Calabrese 2008 [SCA100223] {published data only}
-
- Calabrese JR, Huffman RF, White RL, Edwards S, Thompson TR, Ascher JA, et al. Lamotrigine in the acute treatment of bipolar depression: results of five double-blind, placebo-controlled clinical trials. Bipolar Disorders 2008;10(2):323-33. - PubMed
-
- NCT00274677. Depression and bipolar disorder [A multicenter, double-blind, placebo-controlled, fixed-dose, 8-week evaluation of the efficacy and safety of lamotrigine in the treatment of depression in patients with type II bipolar disorder]. clinicaltrials.gov/ct2/show/NCT00274677 (first received 11 January 2006).
Calabrese 2008 [SCA30924] {published data only}
-
- Calabrese JR, Huffman RF, White RL, Edwards S, Thompson TR, Ascher JA, et al. Lamotrigine in the acute treatment of bipolar depression: results of five double-blind, placebo-controlled clinical trials. Bipolar Disorders 2008;10(2):323-33. - PubMed
-
- NCT00056277. Bipolar disorder study for men and women [Double blind placebo controlled study of lamictal in acute bipolar depression]. clinicaltrials.gov/ct2/show/NCT00056277 (first received 11 March 2003).
Calabrese 2008 [SCA40910] {published data only}
-
- Calabrese JR, Huffman RF, White RL, Edwards S, Thompson TR, Ascher JA, et al. Lamotrigine in the acute treatment of bipolar depression: results of five double-blind, placebo-controlled clinical trials. Bipolar Disorders 2008;10(2):323-33. - PubMed
Koyama 2011 {published data only}
-
- Koyama T, Higuchi T, Yamawaki S, Kanba S, Terao T, Shinohara A. Study SCA104779, an evaluation of BW430C (lamotrigine) versus placebo in the prevention of mood episodes in bipolar Ⅰ disorder patients. Japanese Journal of Clinical Psychiatry 2011;40:369-83.
-
- NCT00550407. An evaluation of BW430C (lamotrigine) versus placebo in the prevention of mood episodes in bipolar I disorder patients. clinicaltrials.gov/ct2/show/NCT00550407 (first received 27 July 2010).
Licht 2010 {published data only}
-
- Licht RW, Nielsen JN, Gram LF, Vestergaard P, Bendz H. Lamotrigine versus lithium as maintenance treatment in bipolar I disorder: an open, randomized effectiveness study mimicking clinical practice. The 6th trial of the Danish University Antidepressant Group (DUAG-6). Bipolar Disorders 2010;12(5):483-93. - PubMed
-
- NCT00226135. Prophylactic effect of lamotrigine compared with lithium in bipolar disorder. clinicaltrials.gov/ct2/show/NCT00226135 (first received 26 September 2005).
References to studies excluded from this review
Berk 1999a {published data only}
-
- Berk M. Lamotrigine and the treatment of mania in bipolar disorder. European Neuropsychopharmacology 1999;9:119-23. - PubMed
Bowden 2012a {published data only}
-
- Bowden CL, Singh V, Weisler R, Thompson P, Chang X, Quinones M, et al. Lamotrigine vs. lamotrigine plus divalproex in randomized, placebo-controlled maintenance treatment for bipolar depression. Acta Psychiatrica Scandinavica 2012;126(5):342-50. - PubMed
ChiCTR2000038166 {published data only}
-
- ChiCTR2000038166. Lithium carbonate and lamotrigine monotherapy or combination therapy as maintenance treatment for childbearing women with bipolar disorder in remission: a randomized controlled study. www.chictr.org.cn/showprojen.aspx?proj=61129 (first received 11 September 2020).
EudraCT 2006‐001317‐15 {published data only}
-
- EudraCT 2006-001317-15. BALANCE 2: Bipolar disorder:Antidepressant/Lamotrigine/ANtipsychotic Comparative Evaluation. www.clinicaltrialsregister.eu/ctr-search/trial/2006-001317-15/GB (first received 9 May 2006).
Frangou 1999 {published data only}
-
- Frangou S. A multicentre, double-blind, placebo-controlled, fixed-dose evaluation of the safety and efficacy of lamotrigine compared to placebo and lithium in the treatment of an acute manic episode in patients who have bipolar disorder: incorporating participants. In: National Research Register. 1999.
Gao 2020 {published data only}
-
- NCT01588457. Sequential multiple assignment treatment for bipolar disorder (SMART) [Sequential multiple assignment randomized treatment (SMART) for bipolar disorder]. clinicaltrials.gov/ct2/show/NCT01588457 (first received 1 May 2012).
Gardiner 2011 {published data only}
-
- Gardiner A, Rendell J, Stephens W, Hainsworth J, Yu LM, Brown S, et al. CEQUEL: A Comparative Evaluation of QUEtiapine-Lamotrigine combination versus quetiapine monotherapy, (and folic acid versus placebo) in people with bipolar depression: A 2x2 factorial randomised trial [conference abstract RC3] [ISRCTN17054996]. In: Bipolar Disorders [Abstracts of the Ninth International Conference on Bipolar Disorder. June 9-11, 2011. Pittsburgh, Pennsylvania, USA]. Vol. 13. 2011:21.
Geddes 2005 {published data only}
-
- Geddes JR, Rendell JM. BALANCE 2: international trial of treatment for bipolar depression. Bipolar Disorders 2005;7:57.
Geddes 2014 {published data only}
-
- Geddes JR, Hinds C, Rendell J, Gardiner A, Voysey M, Attenburrow MJ, et al. Comparative evaluation of quetiapine plus lamotrigine versus quetiapine monotherapy in bipolar depression: a randomized placebo controlled trial (CEQUEL). In: Neuropsychopharmacology. Vol. 39. 2014:S371.
Geddes 2015 {published data only}
-
- Geddes JR, Hinds C, Rendell J, Gardiner A, Voysey M, Attenburrow MJ, et al. Comparative evaluation of quetiapine plus lamotrigine versus quetiapine monotherapy in people with bipolar depression: a randomized trial (CEQUEL). In: Bipolar Disorders. Vol. 17. 2015:25.
Geddes 2015b {published data only}
-
- Geddes JR, Rendell J, Hinds C, Voysey M, Gardiner A, Attenburrow MJ, et al. Comparative evaluation of quetiapine plus lamotrigine versus quetiapine monotherapy in people with bipolar depression: a randomized trial (CEQUEL). Bipolar Disorders 2015;17:50-1.
Geddes 2016 {published data only}
-
- Geddes JR, Gardiner A, Rendell J, Voysey M, Tunbridge E, Hinds C, et al, CEQUEL Investigators and Collaborators. Comparative evaluation of quetiapine plus lamotrigine combination versus quetiapine monotherapy (and folic acid versus placebo) in bipolar depression (CEQUEL): a 2 × 2 factorial randomised trial. Lancet Psychiatry 2016;3(1):31-9. - PubMed
Ketter 2006 {published data only}
-
- Ketter TA, Greist JH, Graham JA, Roberts JN, Thompson TR, Nanry KP. The effect of dermatologic precautions on the incidence of rash with addition of lamotrigine in the treatment of bipolar I disorder: a randomized trial. Journal of Clinical Psychiatry 2006;67(3):400-6. - PubMed
Kwon 2001 {published data only}
-
- Kwon YJ, Jeong HY, Park IJ. Lamotrigine and lithium in the treatment of acute bipolar disorder. Journal of Korean Neuropsychiatric Association 2001;40(5):885-92.
NCT00074776 {published data only}
-
- NCT00074776. Acute treatment of bipolar II depression. clinicaltrials.gov/show/NCT00074776 (first received 22 December 2003).
NCT01195363 {published data only}
-
- NCT01195363. Quetiapine sr as adjunctive treatment in mixed states of bipolar disorder. clinicaltrials.gov/show/NCT01195363 (first received 6 September 2010).
NCT01587066 {published data only}
-
- NCT01587066. Efficacy of quetiapine xr versus divalproex on clinical outcome quality of sleep and quality of life in bipolar depression. clinicaltrials.gov/show/NCT01587066 (first received 27 April 2012).
NCT01674010 {published data only}
-
- NCT01674010. Safety and efficacy study of ELND005 as an adjunctive maintenance treatment in bipolar I disorder. clinicaltrials.gov/show/NCT01674010 (first posted 28 August 2012).
Parikh 2012 {published data only}
-
- Parikh SV, Zaretsky A, Beaulieu S, Yatham LN, Young LT, Patelis-Siotis I, et al. A randomized controlled trial of psychoeducation or cognitive-behavioral therapy in bipolar disorder: a Canadian Network for Mood and Anxiety Treatments (CANMAT) study. Journal of Clinical Psychiatry 2012;73(6):803-10. - PubMed
PER‐122‐12 {published data only}
-
- PER-122-12. A randomized, double-blind, placebo-controlled, phase 3 study to evaluate the efficacy and safety of once a day, tak-375sl 0.1, 0.4, and 0.8 mg as an adjunctive therapy to treatment-as-usual in the maintenance treatment of bipolar 1 disorder in adult subjects. www.who.int/trialsearch/Trial2.aspx?TrialID=PER-122-12.
PER‐123‐12 {published data only}
-
- PER-123-12. A randomized, double-blind, placebo-controlled, phase 3 study to evaluate the efficacy and safety of once a day, tak-375 (ramelteon) tablet for sublingual administration (tak-375sl tablet) 0.1, 04, and 0.8 mg as adjunctive therapy in the treatment of acute depressive episodes associated with bipolar 1 disorder in adult subjects. http://www.who.int/trialsearch/Trial2.aspx?TrialID=PER-123-12.
Simon 2018 {published data only}
Swann 2005 {published data only}
-
- Swann AC. A trial analyzing polypharmacy incorporating extended-release carbamazepine in patients with bipolar I disorder. Bipolar Disorders 2005;7:105.
Tolliver 2014 {published data only}
-
- Tolliver BK. Treatment of co-occurring alcohol dependence and bipolar disorder: preliminary results from a randomized controlled trial of lamotrigine. Alcoholism: Clinical and Experimental Research 2014;38:212.
Tolliver 2018 {published data only}
-
- Tolliver B, Prisciandaro J, Baker N, Brown D, Brenner H, Brady K. A double-blind, placebo-controlled randomized controlled trial of lamotrigine in adults with co-occurring bipolar disorder and alcohol dependence: effects on drinking and mood outcomes. Bipolar Disorders 2018;20:92.
van der Loos 2009 {published data only}
-
- Loos ML, Mulder PG, Hartong EG, Blom MB, Vergouwen AC, Keyzer HJ, et al. Efficacy and safety of lamotrigine as add-on treatment to lithium in bipolar depression: a multicenter, double-blind, placebo-controlled trial. Journal of Clinical Psychiatry 2009;70(2):223-31. - PubMed
van der Loos 2011 {published data only}
-
- Loos ML, Mulder P, Hartong EG, Blom MB, Vergouwen AC, Noorden MS, et al. Long-term outcome of bipolar depressed patients receiving lamotrigine as add-on to lithium with the possibility of the addition of paroxetine in nonresponders: a randomized, placebo-controlled trial with a novel design. Bipolar Disorders 2011;13(1):111-7. - PubMed
Additional references
American Psychiatric Association 2013
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Association. 2013. Available from dsm.psychiatryonline.org/doi/book/10.1176/appi.books.9780890425596.
Andreazza 2014
-
- Andreazza AC, Young LT. The neurobiology of bipolar disorder: identifying targets for specific agents and synergies for combination treatment. International Journal of Neuropsychopharmacology 2014;17(7):1039-52. - PubMed
BALANCE investigators and collaborators 2010
-
- BALANCE investigators and collaborators, Geddes JR, Goodwin GM, Rendell J, Azorin JM, Cipriani A, Ostacher MJ, et al. Lithium plus valproate combination therapy versus monotherapy for relapse prevention in bipolar I disorder (BALANCE): a randomised open-label trial. Lancet 2010;375(9712):385-95. - PubMed
Berk 1999b
-
- Berk M, Ichim L, Brook S. Olanzapine compared to lithium in mania: a double-blind randomized controlled trial. International Clinical Psychopharmacology 1999;14(6):339-43. [PMID: ] - PubMed
Beynon 2009
-
- Beynon S, Soares-Weiser K, Woolacott N, Duffy S, Geddes JR. Pharmacological interventions for the prevention of relapse in bipolar disorder: a systematic review of controlled trials. Journal of Psychopharmacology 2009;23(5):574-91. - PubMed
Blanco 2002
-
- Blanco C, Laje G, Olfson M, Marcus SC, Pincus HA. Trends in the treatment of bipolar disorder by outpatient psychiatrists. American Journal of Psychiatry 2002;159(6):1005-10. [PMID: ] - PubMed
Bobo 2017
-
- Bobo WV. The diagnosis and management of bipolar I and II disorders: clinical practice update. Mayo Clinic Proceedings 2017;92(10):1532-51. [PMID: ] - PubMed
Bowden 2005
-
- Bowden CL, Singh V. Valproate in bipolar disorder: 2000 onwards. Acta Psychiatrica Scandinavica 2005;111(Suppl 426):13-20. [PMID: ] - PubMed
Bowden 2012b
-
- Bowden CL, Singh V. Lamotrigine (Lamictal IR) for the treatment of bipolar disorder. Expert Opinion on Pharmacotherapy 2012;13(17):2565-71. [PMID: ] - PubMed
Calabrese 2005
-
- Calabrese JR, Keck PE Jr, Macfadden W, Minkwitz M, Ketter TA, Weisler RH, et al. A randomized, double-blind, placebo-controlled trial of quetiapine in the treatment of bipolar I or II depression. American Journal of Psychiatry 2005;162(7):1351-60. [PMID: ] - PubMed
Calabrese 2006
-
- Calabrese JR, Goldberg JF, Ketter TA, Suppes T, Frye M, White R, et al. Recurrence in bipolar I disorder: a post hoc analysis excluding relapses in two double-blind maintenance studies. Biological Psychiatry 2006;59(11):1061-4. - PubMed
Calabrese 2008
-
- Calabrese JR, Huffman RF, White RL, Edwards S, Thompson TR, Ascher JA, et al. Lamotrigine in the acute treatment of bipolar depression: results of five double-blind, placebo-controlled clinical trials. Bipolar Disorders 2008;10(2):323-33. [PMID: ] - PubMed
Dauphinais 2011
de Hert 2011
Egger 1997
Garnett 1997
-
- Garnett WR. Lamotrigine: pharmacokinetics. Journal of Child Neurology 1997;12 Suppl 1:S10-5. [PMID: ] - PubMed
Geddes 2009
-
- Geddes JR, Calabrese JR, Goodwin GM. Lamotrigine for treatment of bipolar depression: independent meta-analysis and meta-regression of individual patient data from five randomised trials. British Journal of Psychiatry 2009;194(1):4-9. [PMID: ] - PubMed
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Version accessed 6 July 2021. Hamilton (ON): McMaster University (developed by Evidence Prime), accessed 6 July 2021. Available at gradepro.org.
Graham 2018
-
- Graham RK, Tavella G, Parker GB. Is there consensus across international evidence-based guidelines for the psychotropic drug management of bipolar disorder during the perinatal period? Journal of Affective Disorders 2018;228:216-21. [PMID: ] - PubMed
Greil 1998
-
- Greil W, Kleindienst N, Erazo N, Muller-Oerlinghausen B. Differential response to lithium and carbamazepine in the prophylaxis of bipolar disorder. Journal of Clinical Psychopharmacology 1998;18(6):455-60. [PMID: ] - PubMed
Guyatt 2011
-
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction – GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383-94. [PMID: ] - PubMed
Hamilton 1960
Higgins 2020
-
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews ofInterventions version 6.1 (updated September 2020). Cochrane,2020. Available from www.training.cochrane.org/handbook.
Johannessen 2006
-
- Johannessen SI, Tomson T. Pharmacokinetic variability of newer antiepileptic drugs: when is monitoring needed? Clinical Pharmacokinetics 2006;45(11):1061-75. [PMID: ] - PubMed
Kaufman 2004
-
- Kaufman KR. Monotherapy treatment of bipolar disorder with levetiracetam. Epilepsy & Behavior 2004;5(6):1017-20. [PMID: ] - PubMed
Keck 2003
-
- Keck PE Jr, Marcus R, Tourkodimitris S, Ali M, Liebeskind A, Saha A, et al, Aripiprazole Study Group. A placebo-controlled, double-blind study of the efficacy and safety of aripiprazole in patients with acute bipolar mania. American Journal of Psychiatry 2003;160(9):1651-8. [PMID: ] - PubMed
Ketter 2007
-
- Ketter TA, Jones M, Paulsson B. Rates of remission/euthymia with quetiapine monotherapy compared with placebo in patients with acute mania. Journal of Affective Disorders 2007;100 Suppl 1:S45-53. [PMID: ] - PubMed
Kong 2018
-
- Kong L, Zhou T, Wang B, Gao Z, Wang C. The risks associated with the use of lamotrigine during pregnancy. International Journal of Psychiatry in Clinical Practice 2018;22(1):2-5. [PMID: ] - PubMed
Lyall 2019
Merikangas 2011
Miura 2014
-
- Miura T, Noma H, Furukawa TA, Mitsuyasu H, Tanaka S, Stockton S, et al. Comparative efficacy and tolerability of pharmacological treatments in the maintenance treatment of bipolar disorder: a systematic review and network meta-analysis. Lancet Psychiatry 2014;1(5):351-9. [PMID: ] - PubMed
Moher 2009
-
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of Internal Medicine 2009;151(4):264-9. [PMID: ] - PubMed
Montgomery 1979
-
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979;134(4):382-9. [PMID: ] - PubMed
Murray 2012
-
- Murray CJ, Vos T, Lozano R, Naghavi M, Flaxman AD, Michaud C, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859):2197-223. - PubMed
Ng 2007
Nivoli 2011
-
- Nivoli AM, Colom F, Murru A, Pacchiarotti I, Castro-Loli P, Gonzalez-Pinto A, et al. New treatment guidelines for acute bipolar depression: a systematic review. Journal of Affective Disorders 2011;129(1-3):14-26. [PMID: ] - PubMed
Nordentoft 2011
-
- Nordentoft M, Mortensen PB, Pedersen CB. Absolute risk of suicide after first hospital contact in mental disorder. Archives of General Psychiatry 2011;68(10):1058-64. [PMID: ] - PubMed
Oya 2019
-
- Oya K, Sakuma K, Esumi S, Hashimoto Y, Hatano M, Matsuda Y, et al. Efficacy and safety of lithium and lamotrigine for the maintenance treatment of clinically stable patients with bipolar disorder: a systematic review and meta-analysis of double-blind, randomized, placebo-controlled trials with an enrichment design. Neuropsychopharmacology Reports 2019;39(3):241-6. [PMID: ] - PMC - PubMed
Pariente 2017
-
- Pariente G, Leibson T, Shulman T, Adams-Webber T, Barzilay E, Nulman I. Pregnancy outcomes following in utero exposure to lamotrigine: a systematic review and meta-analysis. CNS Drugs 2017;31(6):439-50. [PMID: ] - PubMed
Pigott 2016
Poels 2018
Rücker 2008
-
- Rücker G, Schwarzer G, Carpenter J. Arcsine test for publication bias in meta-analyses with binary outcomes. Statistics in Medicine 2008;27(5):746-63. [PMID: ] - PubMed
Segal 1998
-
- Segal J, Berk M, Brook S. Risperidone compared with both lithium and haloperidol in mania: a double-blind randomized controlled trial. Clinical Neuropharmacology 1998;21(3):176-80. [PMID: ] - PubMed
Smith 2007
-
- Smith LA, Cornelius V, Warnock A, Bell A, Young AH. Effectiveness of mood stabilizers and antipsychotics in the maintenance phase of bipolar disorder: a systematic review of randomized controlled trials. Bipolar Disorders 2007;9(4):394-412. [PMID: ] - PubMed
Sondergard 2008
-
- Sondergard L, Lopez AG, Andersen PK, Kessing LV. Mood-stabilizing pharmacological treatment in bipolar disorders and risk of suicide. Bipolar Disorders 2008;10(1):87-94. [PMID: ] - PubMed
Suppes 2008b
Vazquez 2015
-
- Vazquez GH, Holtzman JN, Lolich M, Ketter TA, Baldessarini RJ. Recurrence rates in bipolar disorder: systematic comparison of long-term prospective, naturalistic studies versus randomized controlled trials. European Neuropsychopharmacology 2015;25(10):1501-12. [PMID: ] - PubMed
Veroniki 2017
Verrotti 2018
-
- Verrotti A, Striano P, Iapadre G, Zagaroli L, Bonanni P, Coppola G, et al. The pharmacological management of Lennox-Gastaut syndrome and critical literature review. Seizure 2018;63:17-25. [PMID: ] - PubMed
Vieta 2006
-
- Vieta E, Manuel Goikolea J, Martinez-Aran A, Comes M, Verger K, Masramon X, et al. A double-blind, randomized, placebo-controlled, prophylaxis study of adjunctive gabapentin for bipolar disorder. Journal of Clinical Psychiatry 2006;67(3):473-7. [PMID: ] - PubMed
Viguera 2000
-
- Viguera AC, Nonacs R, Cohen LS, Tondo L, Murray A, Baldessarini RJ. Risk of recurrence of bipolar disorder in pregnant and nonpregnant women after discontinuing lithium maintenance. American Journal of Psychiatry 2000;157(2):179-84. [PMID: ] - PubMed
Ware 1993
-
- Ware JE, Snow KK, Kosinski M, Gandek B. SF-36 Health Survey: Manual and Interpretation Guide. Boston (MA): The Health Institute, New England Medical Centre, 1993.
Wesseloo 2017
WHO 2019a
-
- World Health Organization. Mental disorders. www.who.int/news-room/fact-sheets/detail/mental-disorders (accessed 6 July 2021).
WHO 2019b
-
- World Health Organization. ICD-11. International Classification of Diseases 11th Revision. icd.who.int/en (accessed 6 July 2021).
Yatham 2018
-
- Yatham LN, Kennedy SH, Parikh SV, Schaffer A, Bond DJ, Frey BN, et al. Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disorders 2018;20(2)::97-170. - PMC - PubMed
Yildiz 2011
Young 1978
-
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. British Journal of Psychiatry 1978;133:429-35. - PubMed

