Conductive Hearing Loss in the Hyp Mouse Model of X-Linked Hypophosphatemia Is Accompanied by Hypomineralization of the Auditory Ossicles
- PMID: 34523743
- PMCID: PMC8688200
- DOI: 10.1002/jbmr.4443
Conductive Hearing Loss in the Hyp Mouse Model of X-Linked Hypophosphatemia Is Accompanied by Hypomineralization of the Auditory Ossicles
Abstract
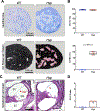
X-linked hypophosphatemia (XLH) is a hereditary musculoskeletal disorder caused by loss-of-function mutations in the PHEX gene. In XLH, increased circulating fibroblast growth factor 23 (FGF23) levels cause renal phosphate wasting and low concentrations of 1,25-dihydroxyvitamin D, leading to an early clinical manifestation of rickets. Importantly, hearing loss is commonly observed in XLH patients. We present here data from two XLH patients with marked conductive hearing loss. To decipher the underlying pathophysiology of hearing loss in XLH, we utilized the Hyp mouse model of XLH and measured auditory brain stem responses (ABRs) and distortion product otoacoustic emissions (DPOAEs) to functionally assess hearing. As evidenced by the increased ABR/DPOAE threshold shifts in the mid-frequency range, these measurements indicated a predominantly conductive hearing loss in Hyp mice compared to wild-type (WT) mice. Therefore, we carried out an in-depth histomorphometric and scanning electron microscopic analysis of the auditory ossicles. Quantitative backscattered electron imaging (qBEI) indicated a severe hypomineralization of the ossicles in Hyp mice, evidenced by lower calcium content (CaMean) and higher void volume (ie, porosity) compared to WT mice. Histologically, voids correlated with unmineralized bone (ie, osteoid), and the osteoid volume per bone volume (OV/BV) was markedly higher in Hyp mice than WT mice. The density of osteocyte lacunae was lower in Hyp mice than in WT mice, whereas osteocyte lacunae were enlarged. Taken together, our findings highlight the importance of ossicular mineralization for hearing conduction and point toward the potential benefit of improving mineralization to prevent hearing loss in XLH. © 2021 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).
Keywords: ANIMAL MODELS; BONE MATRIX; CELL/TISSUE SIGNALING - ENDOCRINE PATHWAYS; GENETIC ANIMAL MODELS; MATRIX MINERALIZATION; PTH/Vit D/FGF23.
© 2021 The Authors. Journal of Bone and Mineral Research published by Wiley Periodicals LLC on behalf of American Society for Bone and Mineral Research (ASBMR).
Conflict of interest statement
Figures




References
-
- Beck-Nielsen SS, Brock-Jacobsen B, Gram J, Brixen K, Jensen TK. Incidence and prevalence of nutritional and hereditary rickets in southern Denmark. Eur J Endocrinol. 2009;160(3):491–7. - PubMed
-
- Liu S, Guo R, Simpson LG, Xiao ZS, Burnham CE, Quarles LD. Regulation of fibroblastic growth factor 23 expression but not degradation by PHEX. J Biol Chem. 2003;278(39):37419–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

