Safety and clinical efficacy of the double switch from originator infliximab to biosimilars CT-P13 and SB2 in patients with inflammatory bowel diseases (SCESICS): A multicenter cohort study
- PMID: 34523800
- PMCID: PMC8742653
- DOI: 10.1111/cts.13131
Safety and clinical efficacy of the double switch from originator infliximab to biosimilars CT-P13 and SB2 in patients with inflammatory bowel diseases (SCESICS): A multicenter cohort study
Abstract
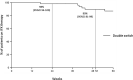
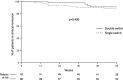
Data regarding double switching from originator infliximab (IFX) to IFX biosimilars in inflammatory bowel diseases (IBDs) are lacking. The purpose of this study was to evaluate the safety and efficacy of switching from originator IFX to CT-P13 and subsequently to SB2 (double switch) in patients with IBD. Patients undergoing IFX-double switch in eight Centers in Lombardy (Italy) from November 2018 to May 2019 were retrospectively analyzed. The IFX discontinuation rate, incidence and type of adverse events (AEs), and clinical remission rate were recorded. A comparison with a control group of patients with IBD single-switched from originator IFX to CT-P13 was performed, before and after an inverse probability of treatment weighting (IPTW)-based propensity score analysis. Fifty-two double-switched patients with IBD were enrolled. The 24- and 52-week proportions of patients continuing on IFX therapy following the second switch (CTP13 → SB2) were 98% (95% confidence interval [CI] 94%-100%) and 90% (95% CI 81%-99%), respectively. Four patients experienced a total of five AEs, all graded 1-3 according to Common Terminology Criteria for Adverse Events (CTCAE). No infusion reactions were observed. The 24-week and follow-up end clinical remission rates following the second switch were 94% and 88%, respectively. No differences were observed in the safety and efficacy outcomes by comparing the double-switch group with a single-switch group of 66 patients with IBD; all these results were confirmed by IPTW-adjusted analysis. The study suggests both the safety and efficacy of the double switch from originator IFX to CT-P13 and SB2 in patients with IBD is maintained. This strategy may be associated with potential cost implications.
© 2021 The Authors. Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declared no competing interests for this work.
Figures

References
-
- Armuzzi A, Fiorino G, Variola A, et al. The PROSIT cohort of infliximab biosimilar in IBD: a prolonged follow‐up on the effectiveness and safety across Italy. Inflamm Bowel Dis. 2019;25(3):568‐579. - PubMed
-
- Fiorino G, Manetti N, Armuzzi A, et al. The PROSIT‐BIO cohort: a prospective observational study of patients with inflammatory bowel disease treated with infliximab biosimilar. Observational Study. Inflamm Bowel Dis. 2017;23(2):233‐243. - PubMed
-
- Ye BD, Pesegova M, Alexeeva O, et al. Efficacy and safety of biosimilar CT‐P13 compared with originator infliximab in patients with active Crohn’s disease: an international, randomised, double‐blind, phase 3 non‐inferiority study. Lancet. 2019;393(10182):1699‐1707. - PubMed
-
- Jørgensen KK, Olsen IC, Goll GL, et al. Switching from originator infliximab to biosimilar CT‐P13 compared with maintained treatment with originator infliximab (NOR‐SWITCH): a 52‐week, randomised, double‐blind, non‐inferiority trial. Lancet. 2017;389(10086):2304‐2316. - PubMed
-
- Choe JY, Prodanovic N, Niebrzydowski J, et al. A randomised, double‐blind, phase III study comparing SB2, an infliximab biosimilar, to the infliximab reference product Remicade in patients with moderate to severe rheumatoid arthritis despite methotrexate therapy. Ann Rheum Dis. 2017;76(1):58‐64. - PMC - PubMed

