Genetic variation in genes regulating skeletal muscle regeneration and tissue remodelling associated with weight loss in chronic obstructive pulmonary disease
- PMID: 34523824
- PMCID: PMC8718068
- DOI: 10.1002/jcsm.12782
Genetic variation in genes regulating skeletal muscle regeneration and tissue remodelling associated with weight loss in chronic obstructive pulmonary disease
Erratum in
-
Correction to 'Genetic variation in genes regulating skeletal muscle regeneration and tissue remodelling associated with weight loss in chronic obstructive pulmonary disease' by Lakshman Kumar et al.J Cachexia Sarcopenia Muscle. 2024 Apr;15(2):765. doi: 10.1002/jcsm.13442. Epub 2024 Feb 7. J Cachexia Sarcopenia Muscle. 2024. PMID: 38327146 Free PMC article. No abstract available.
Abstract
Background: Chronic obstructive pulmonary disease (COPD) is the third leading cause of death globally. COPD patients with cachexia or weight loss have increased risk of death independent of body mass index (BMI) and lung function. We tested the hypothesis genetic variation is associated with weight loss in COPD using a genome-wide association study approach.
Methods: Participants with COPD (N = 4308) from three studies (COPDGene, ECLIPSE, and SPIROMICS) were analysed. Discovery analyses were performed in COPDGene with replication in SPIROMICS and ECLIPSE. In COPDGene, weight loss was defined as self-reported unintentional weight loss > 5% in the past year or low BMI (BMI < 20 kg/m2 ). In ECLIPSE and SPIROMICS, weight loss was calculated using available longitudinal visits. Stratified analyses were performed among African American (AA) and Non-Hispanic White (NHW) participants with COPD. Single variant and gene-based analyses were performed adjusting for confounders. Fine mapping was performed using a Bayesian approach integrating genetic association results with linkage disequilibrium and functional annotation. Significant gene networks were identified by integrating genetic regions associated with weight loss with skeletal muscle protein-protein interaction (PPI) data.
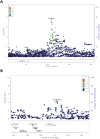
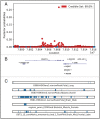
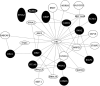
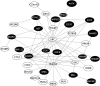
Results: At the single variant level, only the rs35368512 variant, intergenic to GRXCR1 and LINC02383, was associated with weight loss (odds ratio = 3.6, 95% confidence interval = 2.3-5.6, P = 3.2 × 10-8 ) among AA COPD participants in COPDGene. At the gene level in COPDGene, EFNA2 and BAIAP2 were significantly associated with weight loss in AA and NHW COPD participants, respectively. The EFNA2 association replicated among AA from SPIROMICS (P = 0.0014), whereas the BAIAP2 association replicated in NHW from ECLIPSE (P = 0.025). The EFNA2 gene encodes the membrane-bound protein ephrin-A2 involved in the regulation of developmental processes and adult tissue homeostasis such as skeletal muscle. The BAIAP2 gene encodes the insulin-responsive protein of mass 53 kD (IRSp53), a negative regulator of myogenic differentiation. Integration of the gene-based findings participants with PPI data revealed networks of genes involved in pathways such as Rho and synapse signalling.
Conclusions: The EFNA2 and BAIAP2 genes were significantly associated with weight loss in COPD participants. Collectively, the integrative network analyses indicated genetic variation associated with weight loss in COPD may influence skeletal muscle regeneration and tissue remodelling.
Keywords: Biomarkers; COPD; Cachexia; GWAS; Genetics; Skeletal muscle regeneration; Tissue remodelling; Weight loss.
© 2021 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of Society on Sarcopenia, Cachexia and Wasting Disorders.
Conflict of interest statement
R.T‐S. is a former employee and current shareholder of GSK the sponsor of the ECLIPSE study. She declares personal fees from Immunomet, Vocalis Health, Ena Respiratory and Teva. E.K.S. has received institutional grant support from GSK and Bayer. M.W. has received institutional grant support from Mereo BioPharma, Grifols, Vertex Pharmaceuticals, Bayer AG, ARCUS‐Med, Verona, and has been in consultancy roles for AstraZeneca, GlaxoSmithKline, Boehringer Ingelheim, and Takeda. G.R.W. has received institutional grant support from Janssen Pharmaceuticals and Boehringer Ingelheim and has been in consultancy roles for Boehringer Ingelheim, CSL Behring, Janssen Pharmaceuticals, Novartis and Vertex. G.R.W. is also a founder and equity holder of Quantitative Imaging Solutions, a data and image analytics company. W.W.L. reports grants from NIH/NHLBI, non‐financial support from Pulmonx and personal fees from Konica Minolta. M.H.C. has received grant funding from Bayer and GSK and speaking or consulting fees from Genentech, AstraZeneca, and Illumina. H.B.R. received institutional grant support from GlaxoSmithKline, AstraZeneca, and Boehringer Ingelheim and consultancy fees from Boehringer Ingelheim, Astellas Pharma and Omniox Inc. M.T.D. reports grants from NIH, Department of Defense, and the American Lung Association; contracted clinical trial support from AstraZeneca, Boehringer Ingelheim, Boston Scientific, Gala, GlaxoSmithKline, Nuvaira, PneumRx/BTG, and Pulmonx; travel from Pulmonx; and consulting fees from AstraZeneca, GlaxoSmithKline, Mereo, Quark, and Teva.
Figures

References
-
- Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013;187:347–365. - PubMed
-
- Xu J, Murphy SL, Kochanek KD, Bastian B, Arias E. Deaths: final data for 2016. Natl Vital Stat Rep 2018;67:1–76. - PubMed
-
- Evans WJ, Morley JE, Argilés J, Bales C, Baracos V, Guttridge D, et al. Cachexia: a new definition. Clin Nutr 2008;27:793–799. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R56HL153460/NH/NIH HHS/United States
- DH_/Department of Health/United Kingdom
- HHSN268200900018C/HL/NHLBI NIH HHS/United States
- HHSN268200900014C/HL/NHLBI NIH HHS/United States
- P30 DK079626/DK/NIDDK NIH HHS/United States
- HHSN268200900016C/HL/NHLBI NIH HHS/United States
- R01 HL137927/NH/NIH HHS/United States
- HHSN268200900019C/HL/NHLBI NIH HHS/United States
- U01 HL089856/HL/NHLBI NIH HHS/United States
- K08 HL136928/HL/NHLBI NIH HHS/United States
- IK2 RX002165/RX/RRD VA/United States
- HHSN268200900013C/HL/NHLBI NIH HHS/United States
- U01 HL137880/HL/NHLBI NIH HHS/United States
- R00HL121087/NH/NIH HHS/United States
- R01 HL153460/HL/NHLBI NIH HHS/United States
- HHSN268200900017C/HL/NHLBI NIH HHS/United States
- R01HL153460/NH/NIH HHS/United States
- U24 HL141762/HL/NHLBI NIH HHS/United States
- K08 HL136928/NH/NIH HHS/United States
- U01 HL089897/HL/NHLBI NIH HHS/United States
- R01HL135142/NH/NIH HHS/United States
- R01 HL151452/HL/NHLBI NIH HHS/United States
- HHSN268200900020C/HL/NHLBI NIH HHS/United States
- R56 HL153460/HL/NHLBI NIH HHS/United States
- R01 HL137927/HL/NHLBI NIH HHS/United States
- HHSN268200900015C/HL/NHLBI NIH HHS/United States
- R01 HL14714/NH/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

