Grass Carp Reovirus VP56 Allies VP4, Recruits, Blocks, and Degrades RIG-I to More Effectively Attenuate IFN Responses and Facilitate Viral Evasion
- PMID: 34523975
- PMCID: PMC8557896
- DOI: 10.1128/Spectrum.01000-21
Grass Carp Reovirus VP56 Allies VP4, Recruits, Blocks, and Degrades RIG-I to More Effectively Attenuate IFN Responses and Facilitate Viral Evasion
Abstract
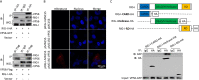
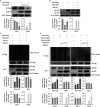
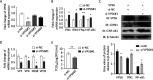
Grass carp reovirus (GCRV), the most virulent aquareovirus, causes epidemic hemorrhagic disease and tremendous economic loss in freshwater aquaculture industry. VP56, a putative fibrin inlaying the outer surface of GCRV-II and GCRV-III, is involved in cell attachment. In the present study, we found that VP56 localizes at the early endosome, lysosome, and endoplasmic reticulum, recruits the cytoplasmic viral RNA sensor retinoic acid-inducible gene I (RIG-I) and binds to it. The interaction between VP56 and RIG-I was detected by endogenous coimmunoprecipitation (co-IP), glutathione S-transferase (GST) pulldown, and subsequent liquid chromatography-tandem mass spectrometry (LC-MS/MS) and was then confirmed by traditional co-IPs and a novel far-red mNeptune-based bimolecular fluorescence complementation system. VP56 binds to the helicase domain of RIG-I. VP56 enhances K48-linked ubiquitination of RIG-I to degrade it by the proteasomal pathway. Thus, VP56 impedes the initial immune function of RIG-I by dual mechanisms (blockade and degradation) and attenuates signaling from RIG-I recognizing viral RNA, subsequently weakening downstream signaling transduction and interferon (IFN) responses. Accordingly, host antiviral effectors are reduced, and cytopathic effects are increased. These findings were corroborated by RNA sequencing (RNA-seq) and VP56 knockdown. Finally, we found that VP56 and the major outer capsid protein VP4 bind together in the cytosol to enhance the degradation of RIG-I and more efficiently facilitate viral replication. Collectively, the results indicated that VP56 allies VP4, recruits, blocks, and degrades RIG-I, thereby attenuating IFNs and antiviral effectors to facilitate viral evasion more effectively. This study reveals a virus attacking target and an escaping strategy from host antiviral immunity for GCRV and will help understand mechanisms of infection of reoviruses. IMPORTANCE Grass carp reovirus (GCRV) fibrin VP56 and major outer capsid protein VP4 inlay and locate on the outer surface of GCRV-II and GCRV-III, which causes tremendous loss in grass carp and black carp industries. Fibrin is involved in cell attachment and plays an important role in reovirus infection. The present study identified the interaction proteins of VP56 and found that VP56 and VP4 bind to the different domains of the viral RNA sensor retinoic acid-inducible gene I (RIG-I) in grass carp to block RIG-I sensing of viral RNA and induce RIG-I degradation by the proteasomal pathway to attenuate signaling transduction, thereby suppressing interferons (IFNs) and antiviral effectors, facilitating viral replication. VP56 and VP4 bind together in the cytosol to more efficiently facilitate viral evasion. This study reveals a virus attacking a target and an escaping strategy from host antiviral immunity for GCRV and will be helpful in understanding the mechanisms of infection of reoviruses.
Keywords: RIG-I; fibrin VP56; grass carp reovirus; host-virus protein interaction; interferon; major outer capsid protein VP4; viral evasion.
Figures







Similar articles
-
Grass carp reovirus VP4 manipulates TOLLIP to degrade STING for inhibition of IFN production.J Virol. 2025 Feb 25;99(2):e0158324. doi: 10.1128/jvi.01583-24. Epub 2025 Jan 14. J Virol. 2025. PMID: 39807855 Free PMC article.
-
Grass Carp Reovirus Major Outer Capsid Protein VP4 Interacts with RNA Sensor RIG-I to Suppress Interferon Response.Biomolecules. 2020 Apr 6;10(4):560. doi: 10.3390/biom10040560. Biomolecules. 2020. PMID: 32268551 Free PMC article.
-
ATG5 negatively regulates grass carp reovirus-induced immune-inflammatory response by degrading RIG-I and MDA5.J Virol. 2025 Jul 22;99(7):e0034425. doi: 10.1128/jvi.00344-25. Epub 2025 Jun 2. J Virol. 2025. PMID: 40454785 Free PMC article.
-
Insights into the antiviral immunity against grass carp (Ctenopharyngodon idella) reovirus (GCRV) in grass carp.J Immunol Res. 2015;2015:670437. doi: 10.1155/2015/670437. Epub 2015 Feb 9. J Immunol Res. 2015. PMID: 25759845 Free PMC article. Review.
-
Host and Viral Modulation of RIG-I-Mediated Antiviral Immunity.Front Immunol. 2017 Jan 3;7:662. doi: 10.3389/fimmu.2016.00662. eCollection 2016. Front Immunol. 2017. PMID: 28096803 Free PMC article. Review.
Cited by
-
Temperature-regulated type II grass carp reovirus establishes latent infection in Ctenopharyngodon idella brain.Virol Sin. 2023 Jun;38(3):440-447. doi: 10.1016/j.virs.2023.04.006. Epub 2023 May 1. Virol Sin. 2023. PMID: 37137379 Free PMC article.
-
Grass carp reovirus VP4 manipulates TOLLIP to degrade STING for inhibition of IFN production.J Virol. 2025 Feb 25;99(2):e0158324. doi: 10.1128/jvi.01583-24. Epub 2025 Jan 14. J Virol. 2025. PMID: 39807855 Free PMC article.
-
Biochemical profiling of the protein encoded by grass carp reovirus genotype II.iScience. 2024 Jul 20;27(8):110502. doi: 10.1016/j.isci.2024.110502. eCollection 2024 Aug 16. iScience. 2024. PMID: 39220409 Free PMC article.
-
Far-Red Fluorescent Proteins: Tools for Advancing In Vivo Imaging.Biosensors (Basel). 2024 Jul 24;14(8):359. doi: 10.3390/bios14080359. Biosensors (Basel). 2024. PMID: 39194588 Free PMC article. Review.
-
Fungal secondary metabolism is governed by an RNA-binding protein CsdA/RsdA complex.Nat Commun. 2023 Nov 14;14(1):7351. doi: 10.1038/s41467-023-43205-2. Nat Commun. 2023. PMID: 37963872 Free PMC article.
References
-
- Mohd Jaafar F, Goodwin AE, Belhouchet M, Merry G, Fang Q, Cantaloube J-F, Biagini P, de Micco P, Mertens PPC, Attoui H. 2008. Complete characterisation of the American grass carp reovirus genome (genus Aquareovirus: family Reoviridae) reveals an evolutionary link between aquareoviruses and coltiviruses. Virology 373:310–321. doi: 10.1016/j.virol.2007.12.006. - DOI - PubMed
-
- Attoui H, Fang Q, Jaafar FM, Cantaloube JF, Biagini P, de Micco P, de Lamballerie X. 2002. Common evolutionary origin of aquareoviruses and orthoreoviruses revealed by genome characterization of Golden shiner reovirus, Grass carp reovirus, Striped bass reovirus and golden ide reovirus (genus Aquareovirus, family Reoviridae). J Gen Virol 83:1941–1951. doi: 10.1099/0022-1317-83-8-1941. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

