Saliva SARS-CoV-2 Antibody Prevalence in Children
- PMID: 34523985
- PMCID: PMC8557814
- DOI: 10.1128/Spectrum.00731-21
Saliva SARS-CoV-2 Antibody Prevalence in Children
Abstract
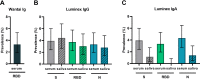
COVID-19 patients produce circulating and mucosal antibodies. In adults, specific saliva antibodies have been detected. Nonetheless, seroprevalence is routinely investigated, while little attention has been paid to mucosal antibodies. We therefore assessed SARS-CoV-2-specific antibody prevalence in serum and saliva in children in the Netherlands. We assessed SARS-CoV-2 antibody prevalence in serum and saliva of 517 children attending medical services in the Netherlands (irrespective of COVID-19 exposure) from April to October 2020. The prevalence of SARS-CoV-2 spike (S), receptor binding domain (RBD), and nucleocapsid (N)-specific IgG and IgA were evaluated with an exploratory Luminex assay in serum and saliva and with the Wantai SARS-CoV-2 RBD total antibody enzyme-linked immunosorbent assay in serum. Using the Wantai assay, the RBD-specific antibody prevalence in serum was 3.3% (95% confidence interval [CI]. 1.9 to 5.3%). With the Luminex assay, we detected heterogeneity between antibodies for S, RBD, and N antigens, as IgG and IgA prevalence ranged between 3.6 and 4.6% in serum and between 0 and 4.4% in saliva. The Luminex assay also revealed differences between serum and saliva, with SARS-CoV-2-specific IgG present in saliva but not in serum for 1.5 to 2.7% of all children. Using multiple antigen assays, the IgG prevalence for at least two out of three antigens (S, RBD, or N) in serum or saliva can be calculated as 3.8% (95% CI, 2.3 to 5.6%). Our study displays the heterogeneity of the SARS-CoV-2 antibody response in children and emphasizes the additional value of saliva antibody detection and the combined use of different antigens. IMPORTANCE Comprehending humoral immunity to SARS-CoV-2, including in children, is crucial for future public health and vaccine strategies. Others have suggested that mucosal antibody measurement could be an important and more convenient tool to evaluate humoral immunity compared to circulating antibodies. Nonetheless, seroprevalence is routinely investigated, while little attention has been paid to mucosal antibodies. We show the heterogeneity of SARS-CoV-2 antibodies, in terms of both antigen specificity and differences between circulating and mucosal antibodies, emphasizing the additional value of saliva antibody detection next to detection of antibodies in serum.
Keywords: SARS-CoV-2; antibodies; children; humoral immunity; prevalence; saliva.
Figures

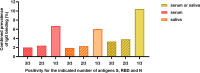
References
-
- World Health Organization. 2020. Population-based age-stratified seroepidemiological investigation protocol for coronavirus 2019 (COVID-19) infection, 26 May 2020. World Health Organization, Geneva, Switzerland.
-
- Pollán M, Pérez-Gómez B, Pastor-Barriuso R, Oteo J, Hernán MA, Pérez-Olmeda M, Sanmartín JL, Fernández-García A, Cruz I, Fernández de Larrea N, Molina M, Rodríguez-Cabrera F, Martín M, Merino-Amador P, León Paniagua J, Muñoz-Montalvo JF, Blanco F, Yotti R, Group E-CS. 2020. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet 396:535–544. doi: 10.1016/S0140-6736(20)31483-5:S0140-6736(20)31483-5. - DOI - PMC - PubMed
-
- Gudbjartsson DF, Norddahl GL, Melsted P, Gunnarsdottir K, Holm H, Eythorsson E, Arnthorsson AO, Helgason D, Bjarnadottir K, Ingvarsson RF, Thorsteinsdottir B, Kristjansdottir S, Birgisdottir K, Kristinsdottir AM, Sigurdsson MI, Arnadottir GA, Ivarsdottir EV, Andresdottir M, Jonsson F, Agustsdottir AB, Berglund J, Eiriksdottir B, Fridriksdottir R, Gardarsdottir EE, Gottfredsson M, Gretarsdottir OS, Gudmundsdottir S, Gudmundsson KR, Gunnarsdottir TR, Gylfason A, Helgason A, Jensson BO, Jonasdottir A, Jonsson H, Kristjansson T, Kristinsson KG, Magnusdottir DN, Magnusson OT, Olafsdottir LB, Rognvaldsson S, Le Roux L, Sigmundsdottir G, Sigurdsson A, Sveinbjornsson G, Sveinsdottir KE, Sveinsdottir M, Thorarensen EA, Thorbjornsson B, Thordardottir M, Saemundsdottir J, et al. 2020. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med 383:1724–1734. doi: 10.1056/NEJMoa2026116. - DOI - PMC - PubMed
-
- Hartley GE, Edwards ESJ, Aui PM, Varese N, Stojanovic S, McMahon J, Peleg AY, Boo I, Drummer HE, Hogarth PM, O’Hehir RE, van Zelm MC. 2020. Rapid generation of durable B cell memory to SARS-CoV-2 spike and nucleocapsid proteins in COVID-19 and convalescence. Sci Immunol 5:eabf8891. doi: 10.1126/sciimmunol.abf8891. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

