Dynamics of the Two Stator Systems in the Flagellar Motor of Pseudomonas aeruginosa Studied by a Bead Assay
- PMID: 34524895
- PMCID: PMC8580004
- DOI: 10.1128/AEM.01674-21
Dynamics of the Two Stator Systems in the Flagellar Motor of Pseudomonas aeruginosa Studied by a Bead Assay
Abstract
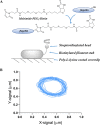
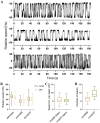
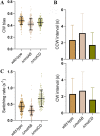
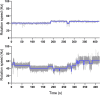
We developed a robust bead assay for studying flagellar motor behavior of Pseudomonas aeruginosa. Using this assay, we studied the dynamics of the two stator systems in the flagellar motor. We found that the two sets of stators function differently, with MotAB stators providing higher total torque and MotCD stators ensuring more stable motor speed. The motors in wild-type cells adjust the stator compositions according to the environment, resulting in an optimal performance in environmental exploration compared to that of mutants with one set of stators. The bead assay we developed in this investigation can be further used to study P. aeruginosa chemotaxis at the level of a single cell using the motor behavior as the chemotaxis output. IMPORTANCE Cells of Pseudomonas aeruginosa possess a single polar flagellum, driven by a rotatory motor powered by two sets of torque-generating units (stators). We developed a robust bead assay for studying the behavior of the flagellar motor in P. aeruginosa, by attaching a microsphere to shortened flagellar filament and using it as an indicator of motor rotation. Using this assay, we revealed the dynamics of the two stator systems in the flagellar motor and found that the motors in wild-type cells adjust the stator compositions according to the environment, resulting in an optimal performance in environmental exploration compared to that of mutants with one set of stators.
Keywords: bead assay; flagellar motor; stator.
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

