Therapeutic effects of high molecular weight hyaluronic acid in severe Pseudomonas aeruginosa pneumonia in ex vivo perfused human lungs
- PMID: 34524905
- PMCID: PMC8616617
- DOI: 10.1152/ajplung.00626.2020
Therapeutic effects of high molecular weight hyaluronic acid in severe Pseudomonas aeruginosa pneumonia in ex vivo perfused human lungs
Abstract
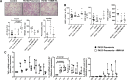
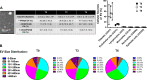
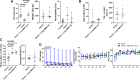
We previously reported that extracellular vesicles (EVs) released during Escherichia coli (E. coli) bacterial pneumonia were inflammatory, and administration of high molecular weight hyaluronic acid (HMW HA) suppressed several indices of acute lung injury (ALI) from E. coli pneumonia by binding to these inflammatory EVs. The current study was undertaken to study the therapeutic effects of HMW HA in ex vivo perfused human lungs injured with Pseudomonas aeruginosa (PA)103 bacterial pneumonia. For lungs with baseline alveolar fluid clearance (AFC) <10%/h, HMW HA 1 or 2 mg was injected intravenously after 1 h (n = 4-9), and EVs released during PA pneumonia were collected from the perfusate over 6 h. For lungs with baseline AFC > 10%/h, HMW HA 2 mg was injected intravenously after 1 h (n = 6). In vitro experiments were conducted to evaluate the effects of HA on inflammation and bacterial phagocytosis. For lungs with AFC < 10%/h, administration of HMW HA intravenously significantly restored AFC and numerically decreased protein permeability and alveolar inflammation from PA103 pneumonia but had no effect on bacterial counts at 6 h. However, HMW HA improved bacterial phagocytosis by human monocytes and neutrophils and suppressed the inflammatory properties of EVs released during pneumonia on monocytes. For lungs with AFC > 10%/h, administration of HMW HA intravenously improved AFC from PA103 pneumonia but had no significant effects on protein permeability, inflammation, or bacterial counts. In the presence of impaired alveolar epithelial transport capacity, administration of HMW HA improved the resolution of pulmonary edema from Pseudomonas PA103 bacterial pneumonia.
Keywords: Pseudomonas aeruginosa pneumonia; acute lung injury; alveolar fluid clearance; extracellular vesicles; hyaluronic acid.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


Similar articles
-
Therapeutic Effects of Hyaluronic Acid in Bacterial Pneumonia in Ex Vivo Perfused Human Lungs.Am J Respir Crit Care Med. 2019 Nov 15;200(10):1234-1245. doi: 10.1164/rccm.201812-2296OC. Am J Respir Crit Care Med. 2019. PMID: 31390880 Free PMC article.
-
Therapeutic Effects of Hyaluronic Acid Against Cytotoxic Extracellular Vesicles Released During Pseudomonas Aeruginosa Pneumonia.Shock. 2022 Mar 1;57(3):408-416. doi: 10.1097/SHK.0000000000001846. Shock. 2022. PMID: 34387224 Free PMC article.
-
Role of CD44 in increasing the potency of mesenchymal stem cell extracellular vesicles by hyaluronic acid in severe pneumonia.Stem Cell Res Ther. 2021 May 20;12(1):293. doi: 10.1186/s13287-021-02329-2. Stem Cell Res Ther. 2021. PMID: 34016170 Free PMC article.
-
Pseudomonas Aeruginosa Induced Cell Death in Acute Lung Injury and Acute Respiratory Distress Syndrome.Int J Mol Sci. 2020 Jul 28;21(15):5356. doi: 10.3390/ijms21155356. Int J Mol Sci. 2020. PMID: 32731491 Free PMC article. Review.
-
The role of extracellular vesicles in traumatic brain injury-induced acute lung injury.Am J Physiol Lung Cell Mol Physiol. 2021 Nov 1;321(5):L885-L891. doi: 10.1152/ajplung.00023.2021. Epub 2021 Sep 22. Am J Physiol Lung Cell Mol Physiol. 2021. PMID: 34549593 Review.
Cited by
-
Administration of sodium hyaluronate to adult horses prior to and immediately after exercise does not alter the range of motion in either the tarsus or metacarpophalangeal joints.Transl Anim Sci. 2024 Nov 2;8:txae153. doi: 10.1093/tas/txae153. eCollection 2024. Transl Anim Sci. 2024. PMID: 39554613 Free PMC article.
-
A Safe-by-Design Approach for the Synthesis of a Novel Cross-Linked Hyaluronic Acid with Improved Biological and Physical Properties.Pharmaceuticals (Basel). 2023 Mar 11;16(3):431. doi: 10.3390/ph16030431. Pharmaceuticals (Basel). 2023. PMID: 36986530 Free PMC article.
-
Respiratory Epithelial Cell Surface Decoration Provides Defense against Bacterial Damage during Infection.Am J Respir Cell Mol Biol. 2024 Dec;71(6):625-627. doi: 10.1165/rcmb.2024-0306ED. Am J Respir Cell Mol Biol. 2024. PMID: 39051864 Free PMC article. No abstract available.
References
-
- Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, Ranieri M, Rubenfeld G, Thompson BT, Wrigge H, Slutsky AS, Pesenti A; LUNG SAFE Investigators, ESICM Trials Group. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 315: 788–800, 2016. doi:10.1001/jama.2016.0291. - DOI - PubMed
-
- Liu L, Yang Y, Gao Z, Li M, Mu X, Ma X, Li G, Sun W, Wang X, Gu Q, Zheng R, Zhao H, Ao D, Yu W, Wang Y, Chen K, Yan J, Li J, Cai G, Wang Y, Wang H, Kang Y, Slutsky AS, Liu S, Xie J, Qiu H. Practice of diagnosis and management of acute respiratory distress syndrome in mainland China: a cross-sectional study. J Thorac Dis 10: 5394–5404, 2018. doi:10.21037/jtd.2018.08.137. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

