Addressing the issue of bias in observational studies: Using instrumental variables and a quasi-randomization trial in an ESME research project
- PMID: 34525119
- PMCID: PMC8443058
- DOI: 10.1371/journal.pone.0255017
Addressing the issue of bias in observational studies: Using instrumental variables and a quasi-randomization trial in an ESME research project
Abstract
Purpose: Observational studies using routinely collected data are faced with a number of potential shortcomings that can bias their results. Many methods rely on controlling for measured and unmeasured confounders. In this work, we investigate the use of instrumental variables (IV) and quasi-trial analysis to control for unmeasured confounders in the context of a study based on the retrospective Epidemiological Strategy and Medical Economics (ESME) database, which compared overall survival (OS) with paclitaxel plus bevacizumab or paclitaxel alone as first-line treatment in patients with HER2-negative metastatic breast cancer (MBC).
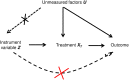
Patients and methods: Causal interpretations and estimates can be made from observation data using IV and quasi-trial analysis. Quasi-trial analysis has the same conceptual basis as IV, however, instead of using IV in the analysis, a "superficial" or "pseudo" randomized trial is used in a Cox model. For instance, in a multicenter trial, instead of using the treatment variable, quasi-trial analysis can consider the treatment preference in each center, which can be informative, and then comparisons of results between centers or clinicians can be informative.
Results: In the original analysis, the OS adjusted for major factors was significantly longer with paclitaxel and bevacizumab than with paclitaxel alone. Using the center-treatment preference as an instrument yielded to concordant results. For the quasi-trial analysis, a Cox model was used, adjusted on all factors initially used. The results consolidate those obtained with a conventional multivariate Cox model.
Conclusion: Unmeasured confounding is a major concern in observational studies, and IV or quasi-trial analysis can be helpful to complement analysis of studies of this nature.
Conflict of interest statement
DP received honoraria from Roche. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
References
-
- Kwiatkowski F, Slim K, Verrelle P, Chamorey E, Kramar A. [Propensity score: Interest and limits]. Bull Cancer (Paris). juill2007;94(7):680–6. - PubMed
-
- D’Agostino RB. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 15oct1998;17(19):2265–81. - PubMed
-
- Martínez-Camblor P, Mackenzie T, Staiger DO, Goodney PP, O’Malley AJ. Adjusting for bias introduced by instrumental variable estimation in the Cox proportional hazards model. Biostatistics [Internet]. 16 déc 2017. [cité 8 oct 2018]; Disponible sur: https://academic.oup.com/biostatistics/advance-article/doi/10.1093/biost... - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous