Safety and Immunogenicity of an Anti-Zika Virus DNA Vaccine
- PMID: 34525286
- PMCID: PMC6824915
- DOI: 10.1056/NEJMoa1708120
Safety and Immunogenicity of an Anti-Zika Virus DNA Vaccine
Abstract
Background: Although Zika virus (ZIKV) infection is typically self-limiting, other associated complications such as congenital birth defects and the Guillain-Barré syndrome are well described. There are no approved vaccines against ZIKV infection.
Methods: In this phase 1, open-label clinical trial, we evaluated the safety and immunogenicity of a synthetic, consensus DNA vaccine (GLS-5700) encoding the ZIKV premembrane and envelope proteins in two groups of 20 participants each. The participants received either 1 mg or 2 mg of vaccine intradermally, with each injection followed by electroporation (the use of a pulsed electric field to introduce the DNA sequence into cells) at baseline, 4 weeks, and 12 weeks.
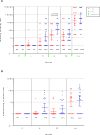
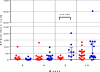
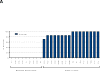
Results: The median age of the participants was 38 years, and 60% were women; 78% were White and 22% Black; in addition, 30% were Hispanic. At the interim analysis at 14 weeks (i.e., after the third dose of vaccine), no serious adverse events were reported. Local reactions at the vaccination site (e.g., injection-site pain, redness, swelling, and itching) occurred in approximately 50% of the participants. After the third dose of vaccine, binding antibodies (as measured on enzyme-linked immunosorbent assay) were detected in all the participants, with geometric mean titers of 1642 and 2871 in recipients of 1 mg and 2 mg of vaccine, respectively. Neutralizing antibodies developed in 62% of the samples on Vero-cell assay. On neuronal-cell assay, there was 90% inhibition of ZIKV infection in 70% of the serum samples and 50% inhibition in 95% of the samples. The intraperitoneal injection of postvaccination serum protected 103 of 112 IFNAR knockout mice (bred with deletion of genes encoding interferon-α and interferon-β receptors) (92%) that were challenged with a lethal dose of ZIKV-PR209 strain; none of the mice receiving baseline serum survived the challenge. Survival was independent of the neutralization titer.
Conclusions: In this phase 1, open-label clinical trial, a DNA vaccine elicited anti-ZIKV immune responses. Further studies are needed to better evaluate the safety and efficacy of the vaccine. (Funded by GeneOne Life Science and others; ZIKA-001 ClinicalTrials.gov number, NCT02809443.).
Copyright © 2017 Massachusetts Medical Society.
Figures




References
-
- Dick G, Kitchen S, Haddow A. Zika virus (I). Isolations and serological specificity. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1952;46:509–20. - PubMed
-
- Duffy MR, Chen TH, Hancock WT, et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. The New England journal of medicine. 2009;360:2536–43. - PubMed
-
- Fauci AS, Morens DM. Zika virus in the Americas—yet another arbovirus threat. New England Journal of Medicine. 2016;374:601–4. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
