Human stem cell-based retina on chip as new translational model for validation of AAV retinal gene therapy vectors
- PMID: 34525384
- PMCID: PMC8452599
- DOI: 10.1016/j.stemcr.2021.08.008
Human stem cell-based retina on chip as new translational model for validation of AAV retinal gene therapy vectors
Abstract
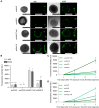
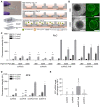
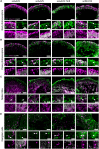
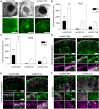
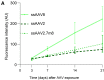
Gene therapies using adeno-associated viruses (AAVs) are among the most promising strategies to treat or even cure hereditary and acquired retinal diseases. However, the development of new efficient AAV vectors is slow and costly, largely because of the lack of suitable non-clinical models. By faithfully recreating structure and function of human tissues, human induced pluripotent stem cell (iPSC)-derived retinal organoids could become an essential part of the test cascade addressing translational aspects. Organ-on-chip (OoC) technology further provides the capability to recapitulate microphysiological tissue environments as well as a precise control over structural and temporal parameters. By employing our recently developed retina on chip that merges organoid and OoC technology, we analyzed the efficacy, kinetics, and cell tropism of seven first- and second-generation AAV vectors. The presented data demonstrate the potential of iPSC-based OoC models as the next generation of screening platforms for future gene therapeutic studies.
Keywords: AAV vectors; gene therapy; human iPSC; organ-on-chip; retina on chip; retinal organoids.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Auricchio A., Behling K.C., Maguire A.M., O’Connor E.E., Bennett J., Wilson J.M., Tolentino M.J. Inhibition of retinal neovascularization by intraocular viral-mediated delivery of anti-angiogenic agents. Mol. Ther. 2002;6:490–494. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

