Biomarkers for autism spectrum disorder: opportunities for magnetoencephalography (MEG)
- PMID: 34525943
- PMCID: PMC8442415
- DOI: 10.1186/s11689-021-09385-y
Biomarkers for autism spectrum disorder: opportunities for magnetoencephalography (MEG)
Abstract
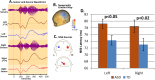
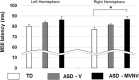
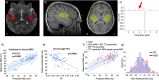
This paper reviews a candidate biomarker for ASD, the M50 auditory evoked response component, detected by magnetoencephalography (MEG) and presents a position on the roles and opportunities for such a biomarker, as well as converging evidence from allied imaging techniques (magnetic resonance imaging, MRI and spectroscopy, MRS). Data is presented on prolonged M50 latencies in ASD as well as extension to include children with ASD with significant language and cognitive impairments in whom M50 latency delays are exacerbated. Modeling of the M50 latency by consideration of the properties of auditory pathway white matter is shown to be successful in typical development but challenged by heterogeneity in ASD; this, however, is capitalized upon to identify a distinct subpopulation of children with ASD whose M50 latencies lie well outside the range of values predictable from the typically developing model. Interestingly, this subpopulation is characterized by low levels of the inhibitory neurotransmitter GABA. Following from this, we discuss a potential use of the M50 latency in indicating "target engagement" acutely with administration of a GABA-B agonist, potentially distinguishing "responders" from "non-responders" with the implication of optimizing inclusion for clinical trials of such agents. Implications for future application, including potential evaluation of infants with genetic risk factors, are discussed. As such, the broad scope of potential of a representative candidate biological marker, the M50 latency, is introduced along with potential future applications.This paper outlines a strategy for understanding brain dysfunction in individuals with intellectual and developmental disabilities (IDD). It is proposed that a multimodal approach (collection of brain structure, chemistry, and neuronal functional data) will identify IDD subpopulations who share a common disease pathway, and thus identify individuals with IDD who might ultimately benefit from specific treatments. After briefly demonstrating the need and potential for scope, examples from studies examining brain function and structure in children with autism spectrum disorder (ASD) illustrate how measures of brain neuronal function (from magnetoencephalography, MEG), brain structure (from magnetic resonance imaging, MRI, especially diffusion MRI), and brain chemistry (MR spectroscopy) can help us better understand the heterogeneity in ASD and form the basis of multivariate biological markers (biomarkers) useable to define clinical subpopulations. Similar approaches can be applied to understand brain dysfunction in neurodevelopmental disorders (NDD) in general. In large part, this paper represents our endeavors as part of the CHOP/Penn NICHD-funded intellectual and developmental disabilities research center (IDDRC) over the past decade.
© 2021. The Author(s).
Conflict of interest statement
Dr. Roberts declares his position on the advisory boards of (1) CTF MEG, (2) Ricoh, (3) Spago Nanomedicine, (4) Avexis Inc., and (5) Acadia Pharmaceuticals and equity interests in (1) Prism Clinical Imaging and (2) Proteus Neurodynamics. Dr. Roberts and Dr. Edgar also declare intellectual property relating to the potential use of electrophysiological markers for treatment planning in clinical ASD. Dr. Edgar declares no other financial conflict. Dr. Kuschner declares no financial conflicts.
Figures

References
-
- Tamminga CA, Pearlson GD, Stan AD, Gibbons RD, Padmanabhan J, Keshavan M, Clementz BA. Strategies for advancing disease definition using biomarkers and genetics: The Bipolar and Schizophrenia Network for Intermediate Phenotypes. Biol Psychiatry Cogn Neurosci Neuroimaging. 2017;2(1):20–27. doi: 10.1016/j.bpsc.2016.07.005. - DOI - PubMed
-
- Turetsky BI, Greenwood TA, Olincy A, Radant AD, Braff DL, Cadenhead KS, Dobie DJ, Freedman R, Green MF, Gur RE, Gur RC, Light GA, Mintz J, Nuechterlein KH, Schork NJ, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, Tsuang DW, Tsuang MT, Calkins ME. Abnormal auditory N100 amplitude: a heritable endophenotype in first-degree relatives of schizophrenia probands. Biol Psychiatry. 2008;64(12):1051–1059. doi: 10.1016/j.biopsych.2008.06.018. - DOI - PMC - PubMed
-
- Chung Y, Allswede D, Addington J, Bearden CE, Cadenhead K, Cornblatt B, Mathalon DH, McGlashan T, Perkins D, Seidman LJ, Tsuang M, Walker E, Woods SW, McEwen S, van Erp T, Cannon TD, North American Prodrome Longitudinal Study (NAPLS) Consortium Cortical abnormalities in youth at clinical high-risk for psychosis: Findings from the NAPLS2 cohort. Neuroimage Clin. 2019;23:101862. doi: 10.1016/j.nicl.2019.101862. - DOI - PMC - PubMed
-
- Wilkinson CL, Gabard-Durnam LJ, Kapur K, Tager-Flusberg H, Levin AR, Nelson CA. Use of longitudinal EEG measures in estimating language development in infants with and without familial risk for autism spectrum disorder. Neurobiol Lang (Camb) 2020;1(1):33–53. doi: 10.1162/nol_a_00002. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

