Clinical characteristics and outcomes of critically ill patients with acute COVID-19 with Epstein-Barr virus reactivation
- PMID: 34525962
- PMCID: PMC8441951
- DOI: 10.1186/s12879-021-06638-y
Clinical characteristics and outcomes of critically ill patients with acute COVID-19 with Epstein-Barr virus reactivation
Abstract
Background: Our goal is to further elucidate the clinical condition and prognosis of patients with severe acute COVID-19 with EBV reactivation.
Method: This is a retrospective single-center study of COVID-19 patients admitted to the intensive care unit of Wuhan No. 3 Hospital (January 31 to March 27, 2020). According to whether Epstein-Barr virus reactivation was detected, the patients were divided into an EBV group and a Non-EBV group. Baseline data were collected including epidemiological, larithmics, clinical and imaging characteristics, and laboratory examination data.
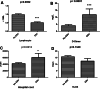
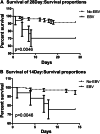
Results: Of the 128 patients with COVID-19, 17 (13.3%) were infected with Epstein-Barr virus reactivation. In the symptoms,the rate of tachypnoea in the EBV group was apparently higher than that in the Non-EBV group. In lab tests, the lymphocyte and albumin of EBV group decreased more significantly than Non-EBV group, and the D-dimer and serum calcium of EBV group was higher than Non-EBV group. Regarding the infection index, CRP of EBV group was apparently above the Non-EBV group, and no significant difference was found in procalcitonin of the two groups. The incidence of respiratory failure, ARDS, and hypoproteinaemia of EBV group had more incidence than Non-EBV group. The 28-day and 14-day mortality rates of EBV group was significantly higher than that of Non-EBV group.
Conclusions: In the COVID-19 patients, patients with EBV reactivation had higher 28-day and 14-day mortality rates and received more immuno-supportive treatment than patients of Non-EBV group.
Keywords: Epstein-Barr virus; Outcome; SARS-CoV-2.
© 2021. The Author(s).
Conflict of interest statement
There are no conflicts of interest.
Figures


References
-
- WHO. Clinical management of severe acute respiratory infection when Novel coronavirus (nCoV) infection is suspected: interim guidance. 2020. https://www.who.int/internal-publications-detail/clinical-management-of-....
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

