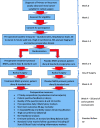
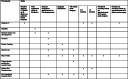
BiCyCLE NMES-neuromuscular electrical stimulation in the perioperative treatment of sarcopenia and myosteatosis in advanced rectal cancer patients: design and methodology of a phase II randomised controlled trial
- PMID: 34526100
- PMCID: PMC8442432
- DOI: 10.1186/s13063-021-05573-2
BiCyCLE NMES-neuromuscular electrical stimulation in the perioperative treatment of sarcopenia and myosteatosis in advanced rectal cancer patients: design and methodology of a phase II randomised controlled trial
Abstract
Background: Colorectal cancer is associated with secondary sarcopenia (muscle loss) and myosteatosis (fatty infiltration of muscle) and patients who exhibit these host characteristics have poorer outcomes following surgery. Furthermore, patients, who undergo curative advanced rectal cancer surgery such as pelvic exenteration, are at risk of skeletal muscle loss due to immobility, malnutrition and a post-surgical catabolic state. Neuromuscular electrical stimulation (NMES) may be a feasible adjunctive treatment to help ameliorate these adverse side-effects. Hence, the purpose of this study is to investigate NMES as an adjunctive pre- and post-operative treatment for rectal cancer patients in the radical pelvic surgery setting and to provide early indicative evidence of efficacy in relation to key health outcomes.
Method: In a phase II, double-blind, randomised controlled study, 58 patients will be recruited and randomised (1:1) to either a treatment (NMES plus standard care) or placebo (sham-NMES plus standard care) group. The intervention will begin 2 weeks pre-operatively and continue for 8 weeks after exenterative surgery. The primary outcome will be change in mean skeletal muscle attenuation, a surrogate marker of myosteatosis. Sarcopenia, quality of life, inflammatory status and cancer specific outcomes will also be assessed.
Discussion: This phase II randomised controlled trial will provide important preliminary evidence of the potential for this adjunctive treatment. It will provide guidance on subsequent development of phase 3 studies on the clinical benefit of NMES for rectal cancer patients in the radical pelvic surgery setting.
Trial registration: Protocol version 6.0; 05/06/20. ClinicalTrials.gov NCT04065984 . Registered on 22 August 2019; recruiting.
Keywords: Advanced rectal cancer; Exenteration surgery; Myosteatosis; NMES; Neuromuscular electrical stimulation; Rehabilitation; Sarcopenia.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures
References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical