Annexin A1 attenuates cardiac diastolic dysfunction in mice with inflammatory arthritis
- PMID: 34526398
- PMCID: PMC8463875
- DOI: 10.1073/pnas.2020385118
Annexin A1 attenuates cardiac diastolic dysfunction in mice with inflammatory arthritis
Abstract
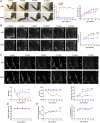
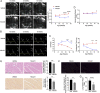
Rheumatoid arthritis (RA) carries a twofold increased incidence of heart failure with preserved ejection fraction, accompanied by diastolic dysfunction, which can lead to death. The causes of diastolic dysfunction are unknown, and there are currently no well-characterized animal models for studying these mechanisms. Current medications for RA do not have marked beneficial cardio-protective effects. K/BxN F1 progeny and KRN control mice were analyzed over time for arthritis development, monitoring left ventricular diastolic and systolic function using echocardiography. Excised hearts were analyzed by flow cytometry, qPCR, and histology. In pharmacological experiments, K/BxN F1 mice were treated with human recombinant AnxA1 (hrAnxA1, 1 μg/mouse) or vehicle daily. K/BxN F1 mice exhibited fully developed arthritis with normal cardiac function at 4 wk; however, by week 8, all mice displayed left ventricular diastolic dysfunction with preserved ejection fraction. This dysfunction was associated with cardiac hypertrophy, myocardial inflammation and fibrosis, and inflammatory markers. Daily treatment of K/BxN F1 mice with hrAnxA1 from weeks 4 to 8 halted progression of the diastolic dysfunction. The treatment reduced cardiac transcripts of proinflammatory cytokines and profibrotic markers. At the cellular level, hrAnxA1 decreased activated T cells and increased MHC IIlow macrophage infiltration in K/BxN F1 hearts. Similar effects were obtained when hrAnxA1 was administered from week 8 to week 15. We describe an animal model of inflammatory arthritis that recapitulates the cardiomyopathy of RA. Treatment with hrAnxA1 after disease onset corrected the diastolic dysfunction through modulation of both fibroblast and inflammatory cell phenotype within the heart.
Keywords: HFpEF; annexin A1; arthritis; cardiomyopathy; diastolic dysfunction.
Copyright © 2021 the Author(s). Published by PNAS.
Conflict of interest statement
Competing interest statement: M.P. is on the Scientific Advisory Board of ResoTher Pharma AS, which is interested in the development of AnxA1-derived peptides for cardiovascular settings. M.P. consults for Bristol Myers Squibb.
Figures






References
-
- Aviña-Zubieta J. A., et al. ., Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Rheum. 59, 1690–1697 (2008). - PubMed
-
- Lazúrová I., Tomáš Ľ., Cardiac impairment in rheumatoid arthritis and influence of anti-TNFα treatment. Clin. Rev. Allergy Immunol. 52, 323–332 (2017). - PubMed
-
- Nicola P. J., et al. ., Contribution of congestive heart failure and ischemic heart disease to excess mortality in rheumatoid arthritis. Arthritis Rheum. 54, 60–67 (2006). - PubMed
-
- Mantel Ä., Holmqvist M., Andersson D. C., Lund L. H., Askling J., Association between rheumatoid arthritis and risk of ischemic and nonischemic heart failure. J. Am. Coll. Cardiol. 69, 1275–1285 (2017). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

