The Glymphatic System: A Novel Component of Fundamental Neurobiology
- PMID: 34526407
- PMCID: PMC8603752
- DOI: 10.1523/JNEUROSCI.0619-21.2021
The Glymphatic System: A Novel Component of Fundamental Neurobiology
Abstract
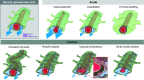
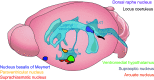
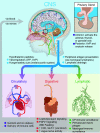
Throughout the body, lymphatic fluid movement supports critical functions including clearance of excess fluid and metabolic waste. The glymphatic system is the analog of the lymphatic system in the CNS. As such, the glymphatic system plays a key role in regulating directional interstitial fluid movement, waste clearance, and, potentially, brain immunity. The glymphatic system enables bulk movement of CSF from the subarachnoid space along periarterial spaces, where it mixes with interstitial fluid within the parenchyma before ultimately exiting from the parenchyma via perivenous spaces. This review focuses on important questions about the structure of this system, why the brain needs a fluid transport system, and unexplored aspects of brain fluid transport. We provide evidence that astrocytes and blood vessels determine the shape of the perivascular space, ultimately controlling the movement of perivascular fluid. Glymphatic fluid movement has the potential to alter local as well as global transport of signaling molecules and metabolites. We also highlight the evidence for cross talk among the glymphatic system, cardiovascular system, gastrointestinal tract, and lymphatic system. Much remains to be studied, but we propose that the glymphatic/lymphatic system acts as a cornerstone in signaling between the brain and body.
Keywords: astrocyte; cerebrospinal fluid; choroid plexus; glymphatic; peptides; perivascular space.
Copyright © 2021 the authors.
Figures

References
-
- Achariyar TM, Li B, Peng W, Verghese PB, Shi Y, McConnell E, Benraiss A, Kasper T, Song W, Takano T, Holtzman DM, Nedergaard M, Deane R (2016) Glymphatic distribution of CSF-derived apoE into brain is isoform specific and suppressed during sleep deprivation. Mol Neurodegener 11:74. 10.1186/s13024-016-0138-8 - DOI - PMC - PubMed
-
- Agnati LF, Fuxe K, Zoli M, Ozini I, Toffano G, Ferraguti F (1986) A correlation analysis of the regional distribution of central enkephalin and beta-endorphin immunoreactive terminals and of opiate receptors in adult and old male rats. Evidence for the existence of two main types of communication in the central nervous system: the volume transmission and the wiring transmission. Acta Physiol Scand 128:201–207. 10.1111/j.1748-1716.1986.tb07967.x - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
