A deep transfer learning approach for wearable sleep stage classification with photoplethysmography
- PMID: 34526643
- PMCID: PMC8443610
- DOI: 10.1038/s41746-021-00510-8
A deep transfer learning approach for wearable sleep stage classification with photoplethysmography
Abstract
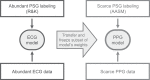
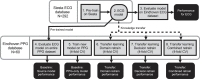
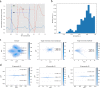
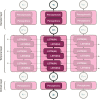
Unobtrusive home sleep monitoring using wrist-worn wearable photoplethysmography (PPG) could open the way for better sleep disorder screening and health monitoring. However, PPG is rarely included in large sleep studies with gold-standard sleep annotation from polysomnography. Therefore, training data-intensive state-of-the-art deep neural networks is challenging. In this work a deep recurrent neural network is first trained using a large sleep data set with electrocardiogram (ECG) data (292 participants, 584 recordings) to perform 4-class sleep stage classification (wake, rapid-eye-movement, N1/N2, and N3). A small part of its weights is adapted to a smaller, newer PPG data set (60 healthy participants, 101 recordings) through three variations of transfer learning. Best results (Cohen's kappa of 0.65 ± 0.11, accuracy of 76.36 ± 7.57%) were achieved with the domain and decision combined transfer learning strategy, significantly outperforming the PPG-trained and ECG-trained baselines. This performance for PPG-based 4-class sleep stage classification is unprecedented in literature, bringing home sleep stage monitoring closer to clinical use. The work demonstrates the merit of transfer learning in developing reliable methods for new sensor technologies by reusing similar, older non-wearable data sets. Further study should evaluate our approach in patients with sleep disorders such as insomnia and sleep apnoea.
© 2021. The Author(s).
Conflict of interest statement
At the time of writing, all authors were employed and/or affiliated with Royal Philips, a commercial company and manufacturer of consumer and medical electronic devices, commercializing products in the area of sleep diagnostics and sleep therapy.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources

