Safety and immunogenicity of SARS-CoV-2 variant mRNA vaccine boosters in healthy adults: an interim analysis
- PMID: 34526698
- PMCID: PMC8604720
- DOI: 10.1038/s41591-021-01527-y
Safety and immunogenicity of SARS-CoV-2 variant mRNA vaccine boosters in healthy adults: an interim analysis
Abstract
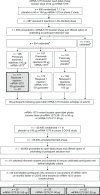
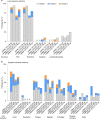
The emergence of SARS-CoV-2 variants of concern (VOCs) and variants of interest (VOIs) with decreased susceptibility to neutralization has generated interest in assessments of booster doses and variant-specific vaccines. Clinical trial participants who received a two-dose primary series of the COVID-19 vaccine mRNA-1273 approximately 6 months earlier entered an open-label phase 2a study ( NCT04405076 ) to evaluate the primary objectives of safety and immunogenicity of a single booster dose of mRNA-1273 or variant-modified mRNAs, including multivalent mRNA-1273.211. As the trial is currently ongoing, this exploratory interim analysis includes preliminary descriptive results only of four booster groups (n = 20 per group). Immediately before the booster dose, neutralizing antibodies against wild-type D614G virus had waned (P < 0.0001) relative to peak titers against wild-type D614G measured 1 month after the primary series, and neutralization titers against B.1.351 (Beta), P.1 (Gamma) and B.1.617.2 (Delta) VOCs were either low or undetectable. Both the mRNA-1273 booster and variant-modified boosters were safe and well-tolerated. All boosters, including mRNA-1273, numerically increased neutralization titers against the wild-type D614G virus compared to peak titers against wild-type D614G measured 1 month after the primary series; significant increases were observed for mRNA-1273 and mRNA-1273.211 (P < 0.0001). In addition, all boosters increased neutralization titers against key VOCs and VOIs, including B.1.351, P.1. and B.1.617.2, that were statistically equivalent to peak titers measured after the primary vaccine series against wild-type D614G virus, with superior titers against some VOIs. This trial is ongoing.
© 2021. The Author(s).
Conflict of interest statement
K.W., A.C., M.K., L.M., T.C., A.H, N.N., W.H., J.O., H.B., H.L., Y.P., B.N., B.D., R.P., A.C., J.M.M., B.L., R.M. and D.K.E. are employees of Moderna and hold stock/stock options in the company. The remaining authors declare no competing interests.
Figures


Comment in
-
Boosting immunity to COVID-19 vaccines.Nat Med. 2021 Nov;27(11):1874-1875. doi: 10.1038/s41591-021-01560-x. Nat Med. 2021. PMID: 34764485 No abstract available.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

