Combined GCMC, MD, and DFT Approach for Unlocking the Performances of COFs for Methane Purification
- PMID: 34526735
- PMCID: PMC8431337
- DOI: 10.1021/acs.iecr.1c01742
Combined GCMC, MD, and DFT Approach for Unlocking the Performances of COFs for Methane Purification
Abstract
Covalent organic frameworks (COFs) are promising materials for gas storage and separation; however, the potential of COFs for separation of CH4 from industrially relevant gases such as H2, N2, and C2H6 is yet to be investigated. In this work, we followed a multiscale computational approach to unlock both the adsorption- and membrane-based CH4/H2, CH4/N2, and C2H6/CH4 separation potentials of 572 COFs by combining grand canonical Monte Carlo (GCMC) and molecular dynamics (MD) simulations and density functional theory (DFT) calculations. Adsorbent performance evaluation metrics of COFs, adsorption selectivity, working capacity, regenerability, and adsorbent performance score were calculated for separation of equimolar CH4/H2, CH4/N2, and C2H6/CH4 mixtures at vacuum swing adsorption (VSA) and pressure swing adsorption (PSA) conditions to identify the best-performing COFs for each mixture. Results showed that COFs could achieve selectivities of 2-85, 1-7, and 2-23 for PSA-based CH4/H2, CH4/N2, and C2H6/CH4 separations, respectively, outperforming conventional adsorbents such as zeolites and activated carbons for each mixture. Structure-performance relations revealed that COFs with pore sizes <10 Å are promising adsorbents for all mixtures. We identified the gas adsorption sites in the three top-performing COFs commonly identified for each mixture by DFT calculations and computed the binding strength of gases, which were found to be on the order of C2H6 > CH4 > N2 > H2, supporting the GCMC results. Nucleus-independent chemical shift (NICS) indexes of aromaticity for adsorption sites were calculated, and the results revealed that the degree of linker aromaticity could be a measure for the selection or design of highly alkane-selective COF adsorbents over N2 and H2. Finally, COF membranes were shown to achieve high H2 permeabilities, 4.57 × 103 -1.25 × 106 Barrer, and decent membrane selectivities, as high as 4.3, outperforming polymeric and MOF-based membranes for separation of H2 from CH4.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



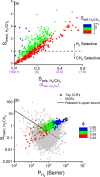
References
-
- Litvinenko V. The Role of Hydrocarbons in the Global Energy Agenda: The Focus on Liquefied Natural Gas. Resources 2020, 9, 5910.3390/resources9050059. - DOI
-
- Bhadra S.; Farooq S. Separation of Methane–Nitrogen Mixture by Pressure Swing Adsorption for Natural Gas Upgrading. Ind. Eng. Chem. Res. 2011, 50, 14030–14045. 10.1021/ie201237x. - DOI
-
- Knaebel S. P.; Ko D.; Biegler L. T. Simulation and Optimization of a Pressure Swing Adsorption System: Recovering Hydrogen from Methane. Adsorption 2005, 11, 615–620. 10.1007/s10450-005-5994-4. - DOI
-
- Olajossy A.; Gawdzik A.; Budner Z.; Dula J. Methane Separation from Coal Mine Methane Gas by Vacuum Pressure Swing Adsorption. Chem. Eng. Res. Des. 2003, 81, 474–482. 10.1205/026387603765173736. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
