Review: Insights on Current FDA-Approved Monoclonal Antibodies Against Ebola Virus Infection
- PMID: 34526994
- PMCID: PMC8435780
- DOI: 10.3389/fimmu.2021.721328
Review: Insights on Current FDA-Approved Monoclonal Antibodies Against Ebola Virus Infection
Abstract
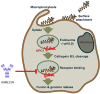
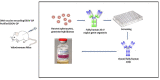
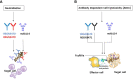
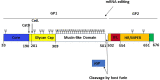
The unprecedented 2013-2016 West Africa Ebola outbreak accelerated several medical countermeasures (MCMs) against Ebola virus disease (EVD). Several investigational products (IPs) were used throughout the outbreak but were not conclusive for efficacy results. Only the Randomized Controlled Trial (RCT) on ZMapp was promising but inconclusive. More recently, during the second-largest Ebola outbreak in North Kivu and Ituri provinces, Democratic Republic of the Congo (DRC), four IPs, including one small molecule (Remdesivir), two monoclonal antibody (mAb) cocktails (ZMapp and REGN-EB3) and a single mAb (mAb114), were evaluated in an RCT, the Pamoja Tulinde Maisha (PALM) study. Two products (REGN-EB3 and mAb114) demonstrated efficacy as compared to the control arm, ZMapp. There were remarkably few side effects recorded in the trial. The FDA approved both medications in this scientifically sound study, marking a watershed moment in the field of EVD therapy. These products can be produced relatively inexpensively and can be stockpiled. The administration of mAbs in EVD patients appears to be safe and effective, while several critical knowledge gaps remain; the impact of early administration of Ebola-specific mAbs on developing a robust immune response for future Ebola virus exposure is unknown. The viral mutation escape, leading to resistance, presents a potential limitation for single mAb therapy; further improvements need to be explored. Understanding the contribution of Fc-mediated antibody functions such as antibody-dependent cellular cytotoxicity (ADCC) of those approved mAbs is still critical. The potential merit of combination therapy and post-exposure prophylaxis (PEP) need to be demonstrated. Furthermore, the PALM trial has accounted for 30% of mortality despite the administration of specific treatments. The putative role of EBOV soluble Glycoprotein (sGP) as a decoy to the immune system, the virus persistence, and relapses might be investigated for treatment failure. The development of pan-filovirus or pan-species mAbs remains essential for protection. The interaction between FDA-approved mAbs and vaccines remains unclear and needs to be investigated. In this review, we summarize the efficacy and safety results of the PALM study and review current research questions for the further development of mAbs in pre-exposure or emergency post-exposure use.
Keywords: Ebola virus; antibodies; filovirus; monoclonal; therapeutics.
Copyright © 2021 Tshiani Mbaya, Mukumbayi and Mulangu.
Conflict of interest statement
OTM is employed by Leidos Biomedical Research. SM is employed by Ridgeback Biotherapeutics, and is listed as inventor on the patent application for mAb 114, US Application No.62/087, 087 (PCT Application No. PCT/US2015/060733) related to anti-Ebola virus antibodies and their use. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- CDC . History of Ebola Virus Disease (EVD) Outbreaks Error Processing SSI File (2021). Available at: https://www.cdc.gov/vhf/ebola/history/chronology.html.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

