Extracellular Vesicles as Novel Diagnostic and Prognostic Biomarkers for Parkinson's Disease
- PMID: 34527424
- PMCID: PMC8407885
- DOI: 10.14336/AD.2021.0527
Extracellular Vesicles as Novel Diagnostic and Prognostic Biomarkers for Parkinson's Disease
Abstract
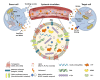
The elderly population will significantly increase in the next decade and, with it, the proportion of people affected by age-related diseases. Among them, one of the most invalidating is Parkinson's disease (PD), characterized by motor- and non-motor dysfunctions which strongly impair the quality of life of affected individuals. PD is characterized by the progressive degeneration of dopaminergic neurons, with consequent dopamine depletion, and the accumulation of misfolded α-synuclein aggregates. Although 150 years have passed since PD first description, no effective therapies are currently available, but only palliative treatments. Importantly, PD is often diagnosed when the neuronal loss is elevated, making difficult any therapeutic intervention. In this context, two key challenges remain unanswered: (i) the early diagnosis to avoid the insurgence of irreversible symptoms; and (ii) the reliable monitoring of therapy efficacy. Research strives to identify novel biomarkers for PD diagnosis, prognosis, and therapeutic follow-up. One of the most promising sources of biomarkers is represented by extracellular vesicles (EVs), a heterogeneous population of nanoparticles, released by all cells in the microenvironment. Brain-derived EVs are able to cross the blood-brain barrier, protecting their payload from enzymatic degradation, and are easily recovered from biofluids. Interestingly, EV content is strongly influenced by the specific pathophysiological status of the donor cell. In this manuscript, the role of EVs as source of novel PD biomarkers is discussed, providing all recent findings concerning relevant proteins and miRNAs carried by PD patient-derived EVs, from several biological specimens. Moreover, the contribution of mitochondria-derived EVs will be dissected. Finally, the promising possibility to use EVs as source of markers to monitor PD therapy efficacy will be also examined. In the future, larger cohort studies will help to validate these EV-associated candidates, that might be effectively used as non-invasive and robust source of biomarkers for PD.
Keywords: Biomarkers; Exosomes; Extracellular Vesicles; Mitochondria-Derived Vesicles (MDVs); Parkinson’s disease; miRNA.
Copyright: © 2021 Leggio et al.
Conflict of interest statement
Conflicts of Interest The authors declare no conflict of interest.
Figures


References
-
- De La Fuente-Fernández R, Schulzer M, Kuramoto L, Cragg J, Ramachandiran N, Au WL, et al.. (2011). Age-specific progression of nigrostriatal dysfunction in Parkinson’s disease. Ann Neurol, 69:803-810. - PubMed
-
- de Lau LM, Breteler MM (2006). Epidemiology of Parkinson’s disease. Lancet Neurol, 5:525-535. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
