Optical ventricular cardioversion by local optogenetic targeting and LED implantation in a cardiomyopathic rat model
- PMID: 34528100
- PMCID: PMC9328286
- DOI: 10.1093/cvr/cvab294
Optical ventricular cardioversion by local optogenetic targeting and LED implantation in a cardiomyopathic rat model
Abstract
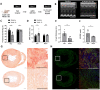
Aims: Ventricular tachyarrhythmias (VTs) are common in the pathologically remodelled heart. These arrhythmias can be lethal, necessitating acute treatment like electrical cardioversion to restore normal rhythm. Recently, it has been proposed that cardioversion may also be realized via optically controlled generation of bioelectricity by the arrhythmic heart itself through optogenetics and therefore without the need of traumatizing high-voltage shocks. However, crucial mechanistic and translational aspects of this strategy have remained largely unaddressed. Therefore, we investigated optogenetic termination of VTs (i) in the pathologically remodelled heart using an (ii) implantable multi-LED device for (iii) in vivo closed-chest, local illumination.
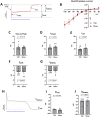
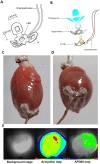
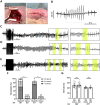
Methods and results: In order to mimic a clinically relevant sequence of events, transverse aortic constriction (TAC) was applied to adult male Wistar rats before optogenetic modification. This modification took place 3 weeks later by intravenous delivery of adeno-associated virus vectors encoding red-activatable channelrhodopsin or Citrine for control experiments. At 8-10 weeks after TAC, VTs were induced ex vivo and in vivo, followed by programmed local illumination of the ventricular apex by a custom-made implanted multi-LED device. This resulted in effective and repetitive VT termination in the remodelled adult rat heart after optogenetic modification, leading to sustained restoration of sinus rhythm in the intact animal. Mechanistically, studies on the single cell and tissue level revealed collectively that, despite the cardiac remodelling, there were no significant differences in bioelectricity generation and subsequent transmembrane voltage responses between diseased and control animals, thereby providing insight into the observed robustness of optogenetic VT termination.
Conclusion: Our results show that implant-based optical cardioversion of VTs is feasible in the pathologically remodelled heart in vivo after local optogenetic targeting because of preserved optical control over bioelectricity generation. These findings add novel mechanistic and translational insight into optical ventricular cardioversion.
Keywords: In vivo; Optogenetics; Remodelling; Ventricular tachycardias.
© The Author(s) 2021. Published by Oxford University Press on behalf of the European Society of Cardiology.
Figures


References
-
- Al-Khatib SM, Stevenson WG, Ackerman MJ, Bryant WJ, Callans DJ, Curtis AB, Deal BJ, Dickfeld T, Field ME, Fonarow GC, Gillis AM, Granger CB, Hammill SC, Hlatky MA, Joglar JA, Kay GN, Matlock DD, Myerburg RJ, Page RL.. 2017 AHA/ACC/HRS guideline for management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: executive summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Heart Rhythm 2018;15:e190–e252. - PubMed
-
- John RM, Tedrow UB, Koplan BA, Albert CM, Epstein LM, Sweeney MO, Miller AL, Michaud GF, Stevenson WG.. Ventricular arrhythmias and sudden cardiac death. Lancet 2012;380:1520–1529. - PubMed
-
- Roberts-Thomson KC, Lau DH, Sanders P.. The diagnosis and management of ventricular arrhythmias. Nat Rev Cardiol 2011;8:311–321. - PubMed
-
- Bradfield JS, Ajijola OA, Vaseghi M, Shivkumar K.. Mechanisms and management of refractory ventricular arrhythmias in the age of autonomic modulation. Heart Rhythm 2018;15:1252–1260. - PubMed
-
- Josephson M, Wellens HJ.. Implantable defibrillators and sudden cardiac death. Circulation 2004;109:2685–2691. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

