Real-World Preliminary Experience With Responsive Neurostimulation in Pediatric Epilepsy: A Multicenter Retrospective Observational Study
- PMID: 34528103
- PMCID: PMC8637802
- DOI: 10.1093/neuros/nyab343
Real-World Preliminary Experience With Responsive Neurostimulation in Pediatric Epilepsy: A Multicenter Retrospective Observational Study
Abstract
Background: Despite the well-documented utility of responsive neurostimulation (RNS, NeuroPace) in adult epilepsy patients, literature on the use of RNS in children is limited.
Objective: To determine the real-world efficacy and safety of RNS in pediatric epilepsy patients.
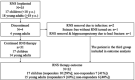
Methods: Patients with childhood-onset drug-resistant epilepsy treated with RNS were retrospectively identified at 5 pediatric centers. Reduction of disabling seizures and complications were evaluated for children (<18 yr) and young adults (>18 yr) and compared with prior literature pertaining to adult patients.
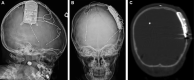
Results: Of 35 patients identified, 17 were <18 yr at the time of RNS implantation, including a 3-yr-old patient. Four patients (11%) had concurrent resection. Three complications, requiring additional surgical interventions, were noted in young adults (2 infections [6%] and 1 lead fracture [3%]). No complications were noted in children. Among the 32 patients with continued therapy, 2 (6%) achieved seizure freedom, 4 (13%) achieved ≥90% seizure reduction, 13 (41%) had ≥50% reduction, 8 (25%) had <50% reduction, and 5 (16%) experienced no improvement. The average follow-up duration was 1.7 yr (median 1.8 yr, range 0.3-4.8 yr). There was no statistically significant difference for seizure reduction and complications between children and young adults in our cohort or between our cohort and the adult literature.
Conclusion: These preliminary data suggest that RNS is well tolerated and an effective off-label surgical treatment of drug-resistant epilepsy in carefully selected pediatric patients as young as 3 yr of age. Data regarding long-term efficacy and safety in children will be critical to optimize patient selection.
Keywords: Brain stimulation; Children; Closed-loop; Eloquent cortex; Multifocal epilepsy; Neuromodulation.
© Congress of Neurological Surgeons 2021.
Figures

References
-
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342(5):314-319. - PubMed
-
- Morrell MJ. Responsive cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77(13):1295-1304. - PubMed
-
- Jobst BC, Kapur R, Barkley GLet al. . Brain-responsive neurostimulation in patients with medically intractable seizures arising from eloquent and other neocortical areas. Epilepsia. 2017;58(6):1005-1014. - PubMed

