Levocarnitine for pegaspargase-induced hepatotoxicity in older children and young adults with acute lymphoblastic leukemia
- PMID: 34528411
- PMCID: PMC8559504
- DOI: 10.1002/cam4.4281
Levocarnitine for pegaspargase-induced hepatotoxicity in older children and young adults with acute lymphoblastic leukemia
Abstract
Background: Pegaspargase (PEG-ASP) is an integral component of therapy for acute lymphoblastic leukemia (ALL) but is associated with hepatotoxicity that may delay or limit future therapy. Obese and adolescent and young adult (AYA) patients are at high risk. Levocarnitine has been described as potentially beneficial for the treatment or prevention of PEG-ASP-associated hepatotoxicity.
Methods: We collected data for patients age ≥10 years who received levocarnitine during induction therapy for ALL, compared to a similar patient cohort who did not receive levocarnitine. The primary endpoint was conjugated bilirubin (c.bili) >3 mg/dl. Secondary endpoints were transaminases >10× the upper limit of normal and any Grade ≥3 hepatotoxicity.
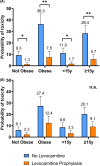
Results: Fifty-two patients received levocarnitine for prophylaxis (n = 29) or rescue (n = 32) of hepatotoxicity. Compared to 109 patients without levocarnitine, more patients receiving levocarnitine were obese and/or older and had significantly higher values for some hepatotoxicity markers at diagnosis and after PEG-ASP. Levocarnitine regimens varied widely; no adverse effects of levocarnitine were identified. Obesity and AYA status were associated with an increased risk of conjugated hyperbilirubinemia and severe transaminitis. Multivariable analysis identified a protective effect of levocarnitine on the development of c.bili >3 mg/dl (OR 0.12, p = 0.029). There was no difference between groups in CTCAE Grade ≥3 hepatotoxicity. C.bili >3 mg/dl during induction was associated with lower event-free survival.
Conclusions: This real-world data on levocarnitine supplementation during ALL induction highlights the risk of PEG-ASP-associated hepatotoxicity in obese and AYA patients, and hepatotoxicity's potential impact on survival. Levocarnitine supplementation may be protective, but prospective studies are needed to confirm these findings.
Keywords: adolescent; asparaginase; carnitine; chemical and drug-induced liver injury; precursor cell lymphoblastic leukemia-lymphoma; young adult.
© 2021 The Authors. Cancer Medicine published by John Wiley & Sons Ltd.
Conflict of interest statement
Advisory board, Jazz Pharmaceuticals (E.O., J.M.); Speaker's bureau, Servier Pharmaceuticals (V.H.)
Figures
Similar articles
-
Levocarnitine for asparaginase-induced hepatic injury: a multi-institutional case series and review of the literature.Leuk Lymphoma. 2018 Oct;59(10):2360-2368. doi: 10.1080/10428194.2018.1435873. Epub 2018 Feb 12. Leuk Lymphoma. 2018. PMID: 29431566 Free PMC article. Review.
-
Levocarnitine for pegasparaginase-induced hepatotoxicity in acute lymphoblastic leukemia.Leuk Lymphoma. 2020 Dec;61(13):3161-3164. doi: 10.1080/10428194.2020.1805108. Epub 2020 Aug 13. Leuk Lymphoma. 2020. PMID: 32787645
-
Levocarnitine and vitamin B complex for the treatment of pegaspargase-induced hepatotoxicity: A case report and review of the literature.J Oncol Pharm Pract. 2018 Jul;24(5):393-397. doi: 10.1177/1078155217710714. Epub 2017 May 19. J Oncol Pharm Pract. 2018. PMID: 28523950 Review.
-
Levocarnitine supplementation for asparaginase-induced hepatotoxicity in adult acute lymphoblastic leukemia patients: A multicenter observational study of the campus all group.Leuk Res. 2022 Nov;122:106963. doi: 10.1016/j.leukres.2022.106963. Epub 2022 Sep 21. Leuk Res. 2022. PMID: 36155352 No abstract available.
-
Pegaspargase-related high-grade hepatotoxicity in a pediatric-inspired adult acute lymphoblastic leukemia regimen does not predict recurrent hepatotoxicity with subsequent doses.Leuk Res. 2018 Mar;66:49-56. doi: 10.1016/j.leukres.2017.12.013. Epub 2018 Jan 3. Leuk Res. 2018. PMID: 29407583 Clinical Trial.
Cited by
-
Hispanic ethnicity and the rs4880 variant in SOD2 are associated with elevated liver enzymes and bilirubin levels in children receiving asparaginase-containing chemotherapy for acute lymphoblastic leukemia.Biomed Pharmacother. 2022 Jun;150:113000. doi: 10.1016/j.biopha.2022.113000. Epub 2022 Apr 29. Biomed Pharmacother. 2022. PMID: 35658244 Free PMC article.
-
The impact of early PEG-asparaginase discontinuation in young adults with ALL: a post hoc analysis of the CALGB 10403 study.Blood Adv. 2023 Jan 24;7(2):196-204. doi: 10.1182/bloodadvances.2022007791. Blood Adv. 2023. PMID: 36269846 Free PMC article.
-
Asparaginase toxicity in Hispanic adult and pediatric patients with acute lymphoblastic leukemia: current understanding.Expert Opin Drug Metab Toxicol. 2023 Jan-Jun;19(6):357-366. doi: 10.1080/17425255.2023.2233412. Expert Opin Drug Metab Toxicol. 2023. PMID: 37410014 Free PMC article. Review.
-
Children's Oncology Group's 2023 blueprint for research: Cancer control and supportive care.Pediatr Blood Cancer. 2023 Sep;70 Suppl 6(Suppl 6):e30568. doi: 10.1002/pbc.30568. Epub 2023 Jul 10. Pediatr Blood Cancer. 2023. PMID: 37430431 Free PMC article.
-
Association of Inherited Genetic Factors With Drug-Induced Hepatic Damage Among Children With Acute Lymphoblastic Leukemia.JAMA Netw Open. 2022 Dec 1;5(12):e2248803. doi: 10.1001/jamanetworkopen.2022.48803. JAMA Netw Open. 2022. PMID: 36580335 Free PMC article.
References
-
- Silverman LB, Gelber RD, Dalton VK, et al. Improved outcome for children with acute lymphoblastic leukemia: results of Dana‐Farber Consortium Protocol 91–01. Blood. 2001;97(5):1211‐1218. - PubMed
-
- Christ TN, Stock W, Knoebel RW. Incidence of asparaginase‐related hepatotoxicity, pancreatitis, and thrombotic events in adults with acute lymphoblastic leukemia treated with a pediatric‐inspired regimen. J Oncol Pharm Pract. 2018;24(4):299‐308. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

