Cefoperazone/sulbactam-induced hemolytic anemia
- PMID: 34528516
- PMCID: PMC9997602
- DOI: 10.4103/jpgm.JPGM_1335_20
Cefoperazone/sulbactam-induced hemolytic anemia
Abstract
Drug-induced hemolytic anemia (DIHA) is a rare complication of drug therapy and usually underdiagnosed. Cefoperazone/sulbactam is a compound prepared from the third generation of cephalosporin and β-lactamase inhibitor. There are limited data of DIHA induced from cefoperazone/sulbactam. A 93-year-old female patient, who had an operation on the biliary tract 3 months ago, was admitted to our hospital with an abdominal infection. After cefoperazone/sulbactam was given as anti-infection treatment, the patient developed hemolytic anemia on the third day. Cefoperazone/sulbactam was discontinued and replaced with meropenem. Subsequently the level of red blood cells, hemoglobin, and hematocrit returned to normal. Clinicians should pay attention to monitoring the possible adverse reactions during the use of cefoperazone/sulbactam and should be aware of the occurrence of DIHA, so as to give timely treatment.
Keywords: Adverse reactions; cefoperazone; drug-related side effects; hemolytic anemia; sulbactam.
Conflict of interest statement
None
Figures
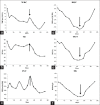
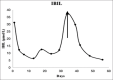
Comment in
-
Drug-induced hemolytic anemia due to cefoperazone-sulbactum: Challenges in reaching diagnosis.J Postgrad Med. 2023 Jan-Mar;69(1):9-10. doi: 10.4103/jpgm.jpgm_248_22. J Postgrad Med. 2023. PMID: 36571331 Free PMC article. No abstract available.
-
The McNamara fallacy in medical education: Spot it, stop it.J Postgrad Med. 2023 Jan-Mar;69(1):7-8. doi: 10.4103/jpgm.jpgm_765_22. J Postgrad Med. 2023. PMID: 36629225 Free PMC article. No abstract available.
References
-
- Iakovlev VP. [Sulperazone--a combined form of cefoperazone with sulbactam] Antibiot Khimioter. 1995;40:55–70. - PubMed
-
- Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239–45. - PubMed
-
- Harcke SJ, Rizzolo D, Harcke HT. G6PD deficiency: An update. JAAPA. 2019;32:21–6. - PubMed
-
- Garratty G. Immune hemolytic anemia associated with drug therapy. Blood Rev. 2010;24:143–50. - PubMed
-
- Arndt PA, Leger RM, Garratty G. Positive direct antiglobulin tests and haemolytic anaemia following therapy with the beta-lactamase inhibitor, tazobactam, may also be associated with non-immunologic adsorption of protein onto red blood cells. Vox Sang. 2003;85:53. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

