Randomised clinical trial: Pemafibrate, a novel selective peroxisome proliferator-activated receptor α modulator (SPPARMα), versus placebo in patients with non-alcoholic fatty liver disease
- PMID: 34528723
- PMCID: PMC9292296
- DOI: 10.1111/apt.16596
Randomised clinical trial: Pemafibrate, a novel selective peroxisome proliferator-activated receptor α modulator (SPPARMα), versus placebo in patients with non-alcoholic fatty liver disease
Abstract
Background: Pemafibrate is a novel, selective peroxisome proliferator-activated receptor α modulator (SPPARMα). In mice, Pemafibrate improved the histological features of non-alcoholic steatohepatitis (NASH). In patients with dyslipidaemia, it improved serum alanine aminotransferase (ALT).
Aims: To evaluate the efficacy and safety of Pemafibrate in patients with high-risk, non-alcoholic fatty liver disease (NAFLD).
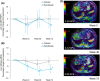
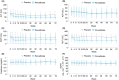
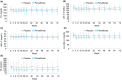
Methods: This double-blind, placebo-controlled, randomised multicentre, phase 2 trial randomised 118 patients (1:1) to either 0.2 mg Pemafibrate or placebo, orally, twice daily for 72 weeks. The key inclusion criteria included liver fat content of ≥10% by magnetic resonance imaging-estimated proton density fat fraction (MRI-PDFF); liver stiffness of ≥2.5 kPa, by magnetic resonance elastography (MRE); and elevated ALT levels. The primary endpoint was the percentage change in MRI-PDFF from baseline to week 24. The secondary endpoints included MRE-based liver stiffness, ALT, serum liver fibrosis markers and lipid parameters.
Results: There was no significant difference between the groups in the primary endpoint (-5.3% vs -4.2%; treatment difference -1.0%, P = 0.85). However, MRE-based liver stiffness significantly decreased compared to placebo at week 48 (treatment difference -5.7%, P = 0.036), and was maintained at week 72 (treatment difference -6.2%, P = 0.024), with significant reduction in ALT and LDL-C. Adverse events were comparable between the treatment groups and therapy was well tolerated.
Conclusions: Pemafibrate did not decrease liver fat content but had significant reduction in MRE-based liver stiffness. Pemafibrate may be a promising therapeutic agent for NAFLD/NASH, and also be a candidate for combination therapy with agents that reduce liver fat content. ClinicalTrials.gov, number: NCT03350165.
© 2021 The Authors. Alimentary Pharmacology & Therapeutics published by John Wiley & Sons Ltd.
Figures
References
-
- Chalasani N, Younossi Z, Lavine Joel E, et al. The diagnosis and management of non‐alcoholic fatty liver disease: practice guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55(6):2005‐2023. - PubMed
-
- National Guideline Centre . NICE Guideline. 2016.
-
- Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease‐Meta‐analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64(1):73‐84. - PubMed
-
- Lonardo A, Nascimbeni F, Mantovani A, et al. Hypertension, diabetes, atherosclerosis and NASH: cause or consequence? J Hepatol. 2018;68(2):335‐352. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
