Mesenchymal stromal cell aging impairs the self-organizing capacity of lung alveolar epithelial stem cells
- PMID: 34528872
- PMCID: PMC8445616
- DOI: 10.7554/eLife.68049
Mesenchymal stromal cell aging impairs the self-organizing capacity of lung alveolar epithelial stem cells
Abstract
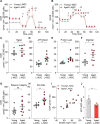
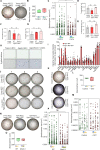
Multicellular organisms maintain structure and function of tissues/organs through emergent, self-organizing behavior. In this report, we demonstrate a critical role for lung mesenchymal stromal cell (L-MSC) aging in determining the capacity to form three-dimensional organoids or 'alveolospheres' with type 2 alveolar epithelial cells (AEC2s). In contrast to L-MSCs from aged mice, young L-MSCs support the efficient formation of alveolospheres when co-cultured with young or aged AEC2s. Aged L-MSCs demonstrated features of cellular senescence, altered bioenergetics, and a senescence-associated secretory profile (SASP). The reactive oxygen species generating enzyme, NADPH oxidase 4 (Nox4), was highly activated in aged L-MSCs and Nox4 downregulation was sufficient to, at least partially, reverse this age-related energy deficit, while restoring the self-organizing capacity of alveolospheres. Together, these data indicate a critical role for cellular bioenergetics and redox homeostasis in an organoid model of self-organization and support the concept of thermodynamic entropy in aging biology.
Keywords: Aging; epithelial stem cells; mesenchymal stromal cells; mouse; oxidative stress; regeneration; regenerative medicine; senescence; stem cells.
Plain language summary
Many tissues in the body are capable of regenerating by replacing defective or worn-out cells with new ones. This process relies heavily on stem cells, which are precursor cells that lack a set role in the body and can develop into different types of cells under the right conditions. Tissues often have their own pool of stem cells that they use to replenish damaged cells. But as we age, this regeneration process becomes less effective. Many of our organs, such as the lungs, are lined with epithelial cells. These cells form a protective barrier, controlling what substances get in and out of the tissue. Alveoli are parts of the lungs that allow oxygen and carbon dioxide to move between the blood and the air in the lungs. And alveoli rely on an effective epithelial cell lining to work properly. To replenish these epithelial cells, alveoli have pockets, in which a type of epithelial cell, known as AEC2, lives. These cells can serve as stem cells, developing into a different type of cell under the right conditions. To work properly, AEC2 cells require close interactions with another type of cell called L-MSC, which supports the maintenance of other cells and also has the ability to differentiate into several other cell types. Both cell types can be found close together in these stem cell pockets. So far, it has been unclear how aging affects how these cells work together to replenish the epithelial lining of the alveoli. To investigate, Chanda et al. probed AEC2s and L-MSCs in the alveoli of young and old mice. The researchers collected both cell types from young (2-3 months) and aged (22-24 months) mice. Various combinations of these cells were grown to form 3D structures, mimicking how the cells grow in the lungs. Young L-MSCs formed normal 3D structures with both young and aged AEC2 cells. But aged L-MSCs developed abnormal, loose structures with AEC2 cells (both young and old cells). Aged L-MSCs were found to have higher levels of an enzyme (called Nox4) that produces oxidants and other ‘pro-aging’ factors, compared to young L-MSCs. However, reducing Nox4 levels in aged L-MSCs allowed these cells to form normal 3D structures with young AEC2 cells, but not aged AEC2 cells. These findings highlight the varying effects specific stem cells have, and how their behaviour is affected by pro-aging factors. Moreover, the pro-aging enzyme Nox4 shows potential as a therapeutic target – downregulating its activity may reverse critical effects of aging in cells.
© 2021, Chanda et al.
Conflict of interest statement
DC, MR, SS, KD, YW, KB, DK, VM, KK, JM, VD, YK, JZ, JD, SD, VT none, GB None
Figures







References
-
- Basil MC, Katzen J, Engler AE, Guo M, Herriges MJ, Kathiriya JJ, Windmueller R, Ysasi AB, Zacharias WJ, Chapman HA, Kotton DN, Rock JR, Snoeck H-W, Vunjak-Novakovic G, Whitsett JA, Morrisey EE. The Cellular and Physiological Basis for Lung Repair and Regeneration: Past, Present, and Future. Cell Stem Cell. 2020;26:482–502. doi: 10.1016/j.stem.2020.03.009. - DOI - PMC - PubMed
-
- Bernard K, Logsdon NJ, Miguel V, Benavides GA, Zhang J, Carter AB, Darley-Usmar VM, Thannickal VJ. NADPH Oxidase 4 (Nox4) Suppresses Mitochondrial Biogenesis and Bioenergetics in Lung Fibroblasts via a Nuclear Factor Erythroid-derived 2-like 2 (Nrf2)-dependent Pathway. The Journal of Biological Chemistry. 2017;292:3029–3038. doi: 10.1074/jbc.M116.752261. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

