Fifteen compelling open questions in plant cell biology
- PMID: 34529074
- PMCID: PMC8774073
- DOI: 10.1093/plcell/koab225
Fifteen compelling open questions in plant cell biology
Abstract
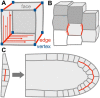
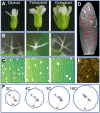
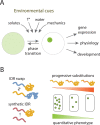
As scientists, we are at least as excited about the open questions-the things we do not know-as the discoveries. Here, we asked 15 experts to describe the most compelling open questions in plant cell biology. These are their questions: How are organelle identity, domains, and boundaries maintained under the continuous flux of vesicle trafficking and membrane remodeling? Is the plant cortical microtubule cytoskeleton a mechanosensory apparatus? How are the cellular pathways of cell wall synthesis, assembly, modification, and integrity sensing linked in plants? Why do plasmodesmata open and close? Is there retrograde signaling from vacuoles to the nucleus? How do root cells accommodate fungal endosymbionts? What is the role of cell edges in plant morphogenesis? How is the cell division site determined? What are the emergent effects of polyploidy on the biology of the cell, and how are any such "rules" conditioned by cell type? Can mechanical forces trigger new cell fates in plants? How does a single differentiated somatic cell reprogram and gain pluripotency? How does polarity develop de-novo in isolated plant cells? What is the spectrum of cellular functions for membraneless organelles and intrinsically disordered proteins? How do plants deal with internal noise? How does order emerge in cells and propagate to organs and organisms from complex dynamical processes? We hope you find the discussions of these questions thought provoking and inspiring.
© The Author(s) 2021. Published by Oxford University Press on behalf of American Society of Plant Biologists.
Figures






Comment in
-
Back to the roots: A focus on plant cell biology.Plant Cell. 2022 Jan 20;34(1):1-3. doi: 10.1093/plcell/koab278. Plant Cell. 2022. PMID: 34755878 Free PMC article. No abstract available.
References
-
- Almonacid M, Terret MÉ, Verlhac MH (2014) Actin-based spindle positioning: new insights from female gametes. J Cell Sci 127: 477–483 - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

