Computational drug repositioning of atorvastatin for ulcerative colitis
- PMID: 34529084
- PMCID: PMC8510297
- DOI: 10.1093/jamia/ocab165
Computational drug repositioning of atorvastatin for ulcerative colitis
Abstract
Objective: Ulcerative colitis (UC) is a chronic inflammatory disorder with limited effective therapeutic options for long-term treatment and disease maintenance. We hypothesized that a multi-cohort analysis of independent cohorts representing real-world heterogeneity of UC would identify a robust transcriptomic signature to improve identification of FDA-approved drugs that can be repurposed to treat patients with UC.
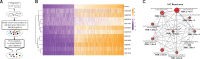
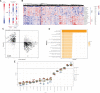
Materials and methods: We performed a multi-cohort analysis of 272 colon biopsy transcriptome samples across 11 publicly available datasets to identify a robust UC disease gene signature. We compared the gene signature to in vitro transcriptomic profiles induced by 781 FDA-approved drugs to identify potential drug targets. We used a retrospective cohort study design modeled after a target trial to evaluate the protective effect of predicted drugs on colectomy risk in patients with UC from the Stanford Research Repository (STARR) database and Optum Clinformatics DataMart.
Results: Atorvastatin treatment had the highest inverse-correlation with the UC gene signature among non-oncolytic FDA-approved therapies. In both STARR (n = 827) and Optum (n = 7821), atorvastatin intake was significantly associated with a decreased risk of colectomy, a marker of treatment-refractory disease, compared to patients prescribed a comparator drug (STARR: HR = 0.47, P = .03; Optum: HR = 0.66, P = .03), irrespective of age and length of atorvastatin treatment.
Discussion & conclusion: These findings suggest that atorvastatin may serve as a novel therapeutic option for ameliorating disease in patients with UC. Importantly, we provide a systematic framework for integrating publicly available heterogeneous molecular data with clinical data at a large scale to repurpose existing FDA-approved drugs for a wide range of human diseases.
Keywords: drug repurposing; gene expression; multi-cohort analysis, ulcerative colitis, electronic health records; transcriptomics.
© The Author(s) 2021. Published by Oxford University Press on behalf of the American Medical Informatics Association.
Figures

References
-
- Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF.. Ulcerative colitis. J Gastroenterol 2018; 53 (5): 585– 1770.
-
- Rubin DT, Ananthakrishnan AN, Siegel CA, Sauer BG, Long MD.. ACG clinical guideline: ulcerative colitis in adults. Am J Gastroenterol 2019; 114 (3): 384–413. - PubMed
-
- Ford AC, Sandborn WJ, Khan KJ, Hanauer SB, Talley NJ, Moayyedi P.. Efficacy of biological therapies in inflammatory bowel disease: systematic review and meta-analysis. Am J Gastroenterol 2011; 106 (4): 644–59; quiz 660. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

