Hyperglycemic conditions proliferate triple negative breast cancer cells: role of ornithine decarboxylase
- PMID: 34529197
- PMCID: PMC8638560
- DOI: 10.1007/s10549-021-06388-0
Hyperglycemic conditions proliferate triple negative breast cancer cells: role of ornithine decarboxylase
Abstract
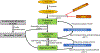
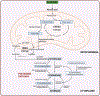
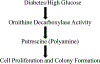
Purpose: Several cancer subtypes (pancreatic, breast, liver, and colorectal) rapidly advance to higher aggressive stages in diabetes. Though hyperglycemia has been considered as a fuel for growth of cancer cells, pathways leading to this condition are still under investigation. Cellular polyamines can modulate normal and cancer cell growth, and inhibitors of polyamine synthesis have been approved for treating colon cancer, however the role of polyamines in diabetes-mediated cancer advancement is unclear as yet. We hypothesized that polyamine metabolic pathway is involved with increased proliferation of breast cancer cells under high glucose (HG) conditions.
Methods: Studies were performed with varying concentrations of glucose (5-25 mM) exposure in invasive, triple negative breast cancer cells, MDA-MB-231; non-invasive, estrogen/progesterone receptor positive breast cancer cells, MCF-7; and non-tumorigenic mammary epithelial cells, MCF-10A.
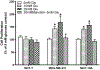
Results: There was a significant increase in proliferation with HG (25 mM) at 48-72 h in both MDA-MB-231 and MCF-10A cells but no such effect was observed in MCF-7 cells. This was correlated to higher activity of ornithine decarboxylase (ODC), a rate-limiting enzyme in polyamine synthesis pathway. Inhibitor of polyamine synthesis (difluoromethylornithine, DFMO, 5 mM) was quite effective in suppressing HG-mediated cell proliferation and ODC activity in MDA-MB-231 and MCF-10A cells. Polyamine (putrescine) levels were significantly elevated with HG treatment in MDA-MB-231 cells. HG exposure also increased the metastasis of MDA-MB-231 cells.
Conclusions: Our cellular findings indicate that polyamine inhibition should be explored in patient population as a target for future chemotherapeutics in diabetic breast cancer.
Keywords: Breast cancer; Diabetes; High glucose; Ornithine decarboxylase; Polyamine; Putrescine.
© 2021. The Author(s), under exclusive licence to Springer Science+Business Media, LLC, part of Springer Nature.
Conflict of interest statement
Declarations of interest: none
Conflicts of interest/Competing interests: None
Figures
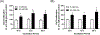



References
-
- Onitilo AA, Stankowski RV, Berg RL, Engel JM, Glurich I, Williams GM, and Doi SA, Breast cancer incidence before and after diagnosis of type 2 diabetes mellitus in women: increased risk in the prediabetes phase. Eur J Cancer Prev, 2014. 23(2): p. 76–83. - PubMed
-
- Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, Pollak M, Regensteiner JG, and Yee D, Diabetes and cancer: a consensus report. CA Cancer J Clin, 2010. 60(4): p. 207–21. - PubMed
-
- Chowdhury TA, Diabetes and cancer. QJM, 2010. 103(12): p. 905–15. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

