Immune correlates of protection by mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates
- PMID: 34529476
- PMCID: PMC8449013
- DOI: 10.1126/science.abj0299
Immune correlates of protection by mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates
Abstract
Immune correlates of protection can be used as surrogate endpoints for vaccine efficacy. Here, nonhuman primates (NHPs) received either no vaccine or doses ranging from 0.3 to 100 μg of the mRNA-1273 severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine. mRNA-1273 vaccination elicited circulating and mucosal antibody responses in a dose-dependent manner. Viral replication was significantly reduced in bronchoalveolar lavages and nasal swabs after SARS-CoV-2 challenge in vaccinated animals and most strongly correlated with levels of anti–S antibody and neutralizing activity. Lower antibody levels were needed for reduction of viral replication in the lower airway than in the upper airway. Passive transfer of mRNA-1273–induced immunoglobulin G to naïve hamsters was sufficient to mediate protection. Thus, mRNA-1273 vaccine–induced humoral immune responses are a mechanistic correlate of protection against SARS-CoV-2 in NHPs.
Figures
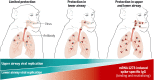
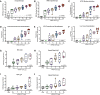


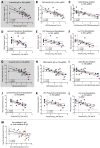

Update of
-
Immune Correlates of Protection by mRNA-1273 Immunization against SARS-CoV-2 Infection in Nonhuman Primates.bioRxiv [Preprint]. 2021 Apr 23:2021.04.20.440647. doi: 10.1101/2021.04.20.440647. bioRxiv. 2021. Update in: Science. 2021 Sep 17;373(6561):eabj0299. doi: 10.1126/science.abj0299. PMID: 33907752 Free PMC article. Updated. Preprint.
References
-
- Pallesen J., Wang N., Corbett K. S., Wrapp D., Kirchdoerfer R. N., Turner H. L., Cottrell C. A., Becker M. M., Wang L., Shi W., Kong W.-P., Andres E. L., Kettenbach A. N., Denison M. R., Chappell J. D., Graham B. S., Ward A. B., McLellan J. S., Immunogenicity and structures of a rationally designed prefusion MERS-CoV spike antigen. Proc. Natl. Acad. Sci. U.S.A. 114, E7348–E7357 (2017). 10.1073/pnas.1707304114 - DOI - PMC - PubMed
-
- Polack F. P., Thomas S. J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J. L., Pérez Marc G., Moreira E. D., Zerbini C., Bailey R., Swanson K. A., Roychoudhury S., Koury K., Li P., Kalina W. V., Cooper D., Frenck R. W. Jr., Hammitt L. L., Türeci Ö., Nell H., Schaefer A., Ünal S., Tresnan D. B., Mather S., Dormitzer P. R., Şahin U., Jansen K. U., Gruber W. C.; C4591001 Clinical Trial Group , Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 383, 2603–2615 (2020). 10.1056/NEJMoa2034577 - DOI - PMC - PubMed
-
- Baden L. R., El Sahly H. M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S. A., Rouphael N., Creech C. B., McGettigan J., Khetan S., Segall N., Solis J., Brosz A., Fierro C., Schwartz H., Neuzil K., Corey L., Gilbert P., Janes H., Follmann D., Marovich M., Mascola J., Polakowski L., Ledgerwood J., Graham B. S., Bennett H., Pajon R., Knightly C., Leav B., Deng W., Zhou H., Han S., Ivarsson M., Miller J., Zaks T.; COVE Study Group , Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 384, 403–416 (2021). 10.1056/NEJMoa2035389 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

