Closed-Loop Neural Prostheses With On-Chip Intelligence: A Review and a Low-Latency Machine Learning Model for Brain State Detection
- PMID: 34529573
- PMCID: PMC8733782
- DOI: 10.1109/TBCAS.2021.3112756
Closed-Loop Neural Prostheses With On-Chip Intelligence: A Review and a Low-Latency Machine Learning Model for Brain State Detection
Abstract
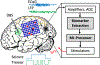
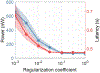
The application of closed-loop approaches in systems neuroscience and therapeutic stimulation holds great promise for revolutionizing our understanding of the brain and for developing novel neuromodulation therapies to restore lost functions. Neural prostheses capable of multi-channel neural recording, on-site signal processing, rapid symptom detection, and closed-loop stimulation are critical to enabling such novel treatments. However, the existing closed-loop neuromodulation devices are too simplistic and lack sufficient on-chip processing and intelligence. In this paper, we first discuss both commercial and investigational closed-loop neuromodulation devices for brain disorders. Next, we review state-of-the-art neural prostheses with on-chip machine learning, focusing on application-specific integrated circuits (ASIC). System requirements, performance and hardware comparisons, design trade-offs, and hardware optimization techniques are discussed. To facilitate a fair comparison and guide design choices among various on-chip classifiers, we propose a new energy-area (E-A) efficiency figure of merit that evaluates hardware efficiency and multi-channel scalability. Finally, we present several techniques to improve the key design metrics of tree-based on-chip classifiers, both in the context of ensemble methods and oblique structures. A novel Depth-Variant Tree Ensemble (DVTE) is proposed to reduce processing latency (e.g., by 2.5× on seizure detection task). We further develop a cost-aware learning approach to jointly optimize the power and latency metrics. We show that algorithm-hardware co-design enables the energy- and memory-optimized design of tree-based models, while preserving a high accuracy and low latency. Furthermore, we show that our proposed tree-based models feature a highly interpretable decision process that is essential for safety-critical applications such as closed-loop stimulation.
Figures





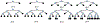

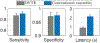
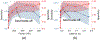
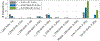


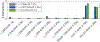
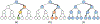

References
-
- Iturrate I, Pereira M, and Millán J. d. R., “Closed-loop electrical neurostimulation: challenges and opportunities,” Current Opinion in Biomedical Engineering, vol. 8, pp. 28–37, 2018.

