Effectiveness of COVID-19 mRNA Vaccines Against COVID-19-Associated Hospitalization - Five Veterans Affairs Medical Centers, United States, February 1-August 6, 2021
- PMID: 34529636
- PMCID: PMC8445376
- DOI: 10.15585/mmwr.mm7037e3
Effectiveness of COVID-19 mRNA Vaccines Against COVID-19-Associated Hospitalization - Five Veterans Affairs Medical Centers, United States, February 1-August 6, 2021
Abstract
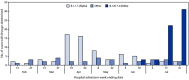
COVID-19 mRNA vaccines (Pfizer-BioNTech and Moderna) have been shown to be highly protective against COVID-19-associated hospitalizations (1-3). Data are limited on the level of protection against hospitalization among disproportionately affected populations in the United States, particularly during periods in which the B.1.617.2 (Delta) variant of SARS-CoV-2, the virus that causes COVID-19, predominates (2). U.S. veterans are older, more racially diverse, and have higher prevalences of underlying medical conditions than persons in the general U.S. population (2,4). CDC assessed the effectiveness of mRNA vaccines against COVID-19-associated hospitalization among 1,175 U.S. veterans aged ≥18 years hospitalized at five Veterans Affairs Medical Centers (VAMCs) during February 1-August 6, 2021. Among these hospitalized persons, 1,093 (93.0%) were men, the median age was 68 years, 574 (48.9%) were non-Hispanic Black (Black), 475 were non-Hispanic White (White), and 522 (44.4%) had a Charlson comorbidity index score of ≥3 (5). Overall adjusted vaccine effectiveness against COVID-19-associated hospitalization was 86.8% (95% confidence interval [CI] = 80.4%-91.1%) and was similar before (February 1-June 30) and during (July 1-August 6) SARS-CoV-2 Delta variant predominance (84.1% versus 89.3%, respectively). Vaccine effectiveness was 79.8% (95% CI = 67.7%-87.4%) among adults aged ≥65 years and 95.1% (95% CI = 89.1%-97.8%) among those aged 18-64 years. COVID-19 mRNA vaccines are highly effective in preventing COVID-19-associated hospitalization in this older, racially diverse population of predominately male U.S. veterans. Additional evaluations of vaccine effectiveness among various age groups are warranted. To prevent COVID-19-related hospitalizations, all eligible persons should receive COVID-19 vaccination.
Conflict of interest statement
All authors have completed and submitted the International Committee of Medical Journal Editors form for disclosure of potential conflicts of interest. Vincent C. Marconi reports research grants from Eli Lilly and Co., Gilead Sciences, and ViiV Healthcare. No other potential conflicts of interest were disclosed.
Figures
References
-
- Tenforde MW, Patel MM, Ginde AA, et al. Effectiveness of SARS-CoV-2 mRNA vaccines for preventing Covid-19 hospitalizations in the United States. Clin Infect Dis 2021. Epub Aug 6, 2021. - PubMed
-
- Young-Xu Y, Korves C, Roberts J, et al. Coverage and effectiveness of mRNA COVID-19 vaccines among veterans. medRxiv [Preprint posted online July 14, 2021]. https://www.medrxiv.org/content/10.1101/2021.06.14.21258906v3 - DOI - PMC - PubMed
-
- King JT Jr, Yoon JS, Rentsch CT, et al. Development and validation of a 30-day mortality index based on pre-existing medical administrative data from 13,323 COVID-19 patients: The Veterans Health Administration COVID-19 (VACO) Index. PLoS One 2020;15:e0241825. 10.1371/journal.pone.0241825 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

