A high-frequency single nucleotide polymorphism in the MtrB sensor kinase in clinical strains of Mycobacterium tuberculosis alters its biochemical and physiological properties
- PMID: 34529706
- PMCID: PMC8445491
- DOI: 10.1371/journal.pone.0256664
A high-frequency single nucleotide polymorphism in the MtrB sensor kinase in clinical strains of Mycobacterium tuberculosis alters its biochemical and physiological properties
Abstract
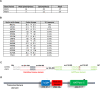
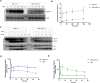
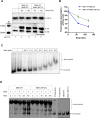
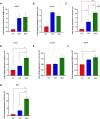
The DNA polymorphisms found in clinical strains of Mycobacterium tuberculosis drive altered physiology, virulence, and pathogenesis in them. Although the lineages of these clinical strains can be traced back to common ancestor/s, there exists a plethora of difference between them, compared to those that have evolved in the laboratory. We identify a mutation present in ~80% of clinical strains, which maps in the HATPase domain of the sensor kinase MtrB and alters kinase and phosphatase activities, and affects its physiological role. The changes conferred by the mutation were probed by in-vitro biochemical assays which revealed changes in signaling properties of the sensor kinase. These changes also affect bacterial cell division rates, size and membrane properties. The study highlights the impact of DNA polymorphisms on the pathophysiology of clinical strains and provides insights into underlying mechanisms that drive signal transduction in pathogenic bacteria.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Manca C, Tsenova L, Iii CEB, Freeman S, Haslett PAJ, James M, et al.. Mycobacterium tuberculosis CDC1551 Induces a More Vigorous Host Response In Vivo and In Vitro, But Is Not More Virulent Than Other Clinical Isolates. J Immunol. 1999;162: 6740–6746. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

