PKM2 promotes neutrophil activation and cerebral thromboinflammation: therapeutic implications for ischemic stroke
- PMID: 34529778
- PMCID: PMC8874361
- DOI: 10.1182/blood.2021012322
PKM2 promotes neutrophil activation and cerebral thromboinflammation: therapeutic implications for ischemic stroke
Abstract
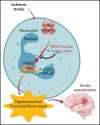
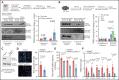
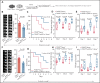
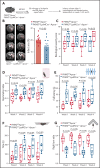
There is a critical need for cerebro-protective interventions to improve the suboptimal outcomes of patients with ischemic stroke who have been treated with reperfusion strategies. We found that nuclear pyruvate kinase muscle 2 (PKM2), a modulator of systemic inflammation, was upregulated in neutrophils after the onset of ischemic stroke in both humans and mice. Therefore, we determined the role of PKM2 in stroke pathogenesis by using murine models with preexisting comorbidities. We generated novel myeloid cell-specific PKM2-/- mice on wild-type (PKM2fl/flLysMCre+) and hyperlipidemic background (PKM2fl/flLysMCre+Apoe-/-). Controls were littermate PKM2fl/flLysMCre- or PKM2fl/flLysMCre-Apoe-/- mice. Genetic deletion of PKM2 in myeloid cells limited inflammatory response in peripheral neutrophils and reduced neutrophil extracellular traps after cerebral ischemia and reperfusion, suggesting that PKM2 promotes neutrophil hyperactivation in the setting of stroke. In the filament and autologous clot and recombinant tissue plasminogen activator stroke models, irrespective of sex, deletion of PKM2 in myeloid cells in either wild-type or hyperlipidemic mice reduced infarcts and enhanced long-term sensorimotor recovery. Laser speckle imaging revealed improved regional cerebral blood flow in myeloid cell-specific PKM2-deficient mice that was concomitant with reduced post-ischemic cerebral thrombo-inflammation (intracerebral fibrinogen, platelet [CD41+] deposition, neutrophil infiltration, and inflammatory cytokines). Mechanistically, PKM2 regulates post-ischemic inflammation in peripheral neutrophils by promoting STAT3 phosphorylation. To enhance the translational significance, we inhibited PKM2 nuclear translocation using a small molecule and found significantly reduced neutrophil hyperactivation and improved short-term and long-term functional outcomes after stroke. Collectively, these findings identify PKM2 as a novel therapeutic target to improve brain salvage and recovery after reperfusion.
© 2022 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Figures
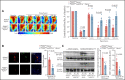
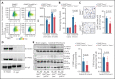
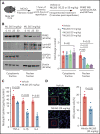

Comment in
-
Targeting neutrophil PKM2 for stroke treatment.Blood. 2022 Feb 24;139(8):1131-1132. doi: 10.1182/blood.2021014199. Blood. 2022. PMID: 35201329 No abstract available.
References
-
- Alexandrov AV, Grotta JC. Arterial reocclusion in stroke patients treated with intravenous tissue plasminogen activator. Neurology. 2002;59(6):862-867. - PubMed
-
- Rubiera M, Alvarez-Sabín J, Ribo M, et al. . Predictors of early arterial reocclusion after tissue plasminogen activator-induced recanalization in acute ischemic stroke. Stroke. 2005;36(7):1452-1456. - PubMed
-
- Goyal M, Menon BK, van Zwam WH, et al. ; HERMES collaborators . Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387(10029):1723-1731. - PubMed
-
- Stoll G, Nieswandt B. Thrombo-inflammation in acute ischaemic stroke - implications for treatment. Nat Rev Neurol. 2019;15(8):473-481. - PubMed
-
- De Meyer SF, Denorme F, Langhauser F, Geuss E, Fluri F, Kleinschnitz C. Thromboinflammation in stroke brain damage. Stroke. 2016;47(4): 1165-1172. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

