First description of immune complex vasculitis after COVID-19 vaccination with BNT162b2: a case report
- PMID: 34530771
- PMCID: PMC8443965
- DOI: 10.1186/s12879-021-06655-x
First description of immune complex vasculitis after COVID-19 vaccination with BNT162b2: a case report
Abstract
Background: Cases of immune complex vasculitis have been reported following COVID-19 infections; so far none in association with novel mRNA-based COVID-19 vaccination. This case report describes a cutaneous immune complex vasculitis after vaccination with BNT162b2.
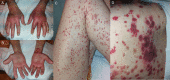
Case presentation: A 76-year old male with liver cirrhosis developed an immune complex vasculitis 12 days after the second injection of BNT162b2. On physical examination, the patient presented with pruritic purpuric macules on hands and feet, flexor and extensor parts of both legs and thighs and lower abdomen, and bloody diarrhoea. Laboratory testing showed elevated inflammatory markers. After short treatment with oral steroids all clinical manifestations and laboratory findings resolved.
Conclusions: An increasing number of clinical manifestations have been attributed to COVID-19 infection and vaccination. This is the first written report of immune complex vasculitis after vaccination with BNT162b2. We present our case report and a discussion in the light of type three hypersensitivity reaction.
Keywords: BNT162b2; COVID-19 vaccination; Case report; Immune complex vasculitis; Type three hypersensitivity.
© 2021. The Author(s).
Conflict of interest statement
VTM: None; VK: None; MMM: None; FO: None; SZ: None.
Figures

References
-
- World Health Organization. Coronavirus diseaese (COVID-19) pandemic. (https://www.who.int/emergencies/diseases/novel-coronavirus-2019, Last accessed: 05/20/2021).
-
- John Hopkins University & Medicine - Corona Virus Resource Center. (https://coronavirus.jhu.edu/, Last accessed 05/20/2021).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

