Engineering the substrate binding site of the hyperthermostable archaeal endo-β-1,4-galactanase from Ignisphaera aggregans
- PMID: 34530892
- PMCID: PMC8447715
- DOI: 10.1186/s13068-021-02025-6
Engineering the substrate binding site of the hyperthermostable archaeal endo-β-1,4-galactanase from Ignisphaera aggregans
Abstract
Background: Endo-β-1,4-galactanases are glycoside hydrolases (GH) from the GH53 family belonging to the largest clan of GHs, clan GH-A. GHs are ubiquitous and involved in a myriad of biological functions as well as being widely used industrially. Endo-β-1,4-galactanases, in particular hydrolyse galactan and arabinogalactan in pectin, a major component of the primary plant cell wall, with important functions in plant defence and application in the food and other industries. Here, we explore the family's biological diversity by characterizing the first archaeal and hyperthermophilic GH53 galactanase, and utilize it as a scaffold for engineering enzymes with different product lengths.
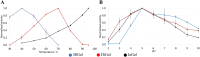
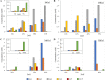
Results: A galactanase gene was identified in the genome of the anaerobic hyperthermophilic archaeon Ignisphaera aggregans, and the isolated catalytic domain expressed and characterized (IaGal). IaGal presents the typical (βα)8 barrel structure of clan GH-A enzymes, with catalytic carboxylates at the end of the 4th and 7th barrel strands. Its activity optimum of at least 95 °C and melting point over 100 °C indicate extreme thermostability, a very advantageous property for industrial applications. If enzyme depletion is reduced, so is the need for re-addition, and thus costs. The main stabilizing features of IaGal compared to other structurally characterized members are π-π and cation-π interactions. The length of the substrate binding site-and thus produced oligosaccharide products-is intermediate compared to previously characterized galactanases. Variants inspired by the structural diversity in the GH53 family were rationally designed to shorten or extend the substrate binding groove, in order to modulate product length. Subsite-deleted variants produced shorter products than IaGal, as do the fungal galactanases inspiring the design. IaGal variants engineered with a longer binding site produced a less expected degradation pattern, though still different from that of wild-type IaGal. All variants remained extremely stable.
Conclusions: We have characterized in detail the most thermophilic endo-β-1,4-galactanase known to date and successfully engineered it to modify the degradation profile, while maintaining much of its desirable thermostability. This is an important achievement as oligosaccharide products length is an important property for industrial and natural GHs alike.
Keywords: Archaea; Biomass degradation; Crystal structure; Degradation profiles; Extreme thermophile; Galactan; Glycoside hydrolase; High-performance anion-exchange chromatography; Ignisphaera aggregans; Rational design.
© 2021. The Author(s).
Conflict of interest statement
The authors declare the following conflicts of interest: KBRMK and KJ work for Novozymes, a major manufacturer of industrial enzymes.
Figures







References
-
- Lau JM, McNeil M, Darvill AG, Albersheim P. Structure of the backbone of rhamnogalacturonan I, a pectic polysaccharide in the primary cell walls of plants. Carbohydr Res. 1985;137 C:111–125. doi: 10.1016/0008-6215(85)85153-3. - DOI
-
- Voragen AGJ, Coenen G-J, Verhoef RP, Schols HA. Pectin, a versatile polysaccharide present in plant cell walls. Struct Chem. 2009;20:263–275. doi: 10.1007/s11224-009-9442-z. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

