Blood biomarkers for mild traumatic brain injury: a selective review of unresolved issues
- PMID: 34530937
- PMCID: PMC8447604
- DOI: 10.1186/s40364-021-00325-5
Blood biomarkers for mild traumatic brain injury: a selective review of unresolved issues
Abstract
Background: The use of blood biomarkers after mild traumatic brain injury (mTBI) has been widely studied. We have identified eight unresolved issues related to the use of five commonly investigated blood biomarkers: neurofilament light chain, ubiquitin carboxy-terminal hydrolase-L1, tau, S100B, and glial acidic fibrillary protein. We conducted a focused literature review of unresolved issues in three areas: mode of entry into and exit from the blood, kinetics of blood biomarkers in the blood, and predictive capacity of the blood biomarkers after mTBI.
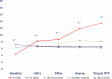
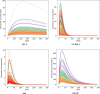
Findings: Although a disruption of the blood brain barrier has been demonstrated in mild and severe traumatic brain injury, biomarkers can enter the blood through pathways that do not require a breach in this barrier. A definitive accounting for the pathways that biomarkers follow from the brain to the blood after mTBI has not been performed. Although preliminary investigations of blood biomarkers kinetics after TBI are available, our current knowledge is incomplete and definitive studies are needed. Optimal sampling times for biomarkers after mTBI have not been established. Kinetic models of blood biomarkers can be informative, but more precise estimates of kinetic parameters are needed. Confounding factors for blood biomarker levels have been identified, but corrections for these factors are not routinely made. Little evidence has emerged to date to suggest that blood biomarker levels correlate with clinical measures of mTBI severity. The significance of elevated biomarker levels thirty or more days following mTBI is uncertain. Blood biomarkers have shown a modest but not definitive ability to distinguish concussed from non-concussed subjects, to detect sub-concussive hits to the head, and to predict recovery from mTBI. Blood biomarkers have performed best at distinguishing CT scan positive from CT scan negative subjects after mTBI.
Keywords: Blood biomarkers; CT scan; Concussion; GFAP; Kinetics; Mild traumatic brain injury; NF-L; Return to sport; S100B; Tau; UCH-L1.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

