Side Effect Patterns in a Crossover Trial of Statin, Placebo, and No Treatment
- PMID: 34531021
- PMCID: PMC8453640
- DOI: 10.1016/j.jacc.2021.07.022
Side Effect Patterns in a Crossover Trial of Statin, Placebo, and No Treatment
Abstract
Background: Most people who begin statins abandon them, most commonly because of side effects.
Objectives: The purpose of this study was to assess daily symptom scores on statin, placebo, and no treatment in participants who had abandoned statins.
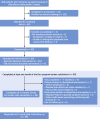
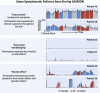
Methods: Participants received 12 1-month medication bottles, 4 containing atorvastatin 20 mg, 4 placebo, and 4 empty. We measured daily symptom intensity for each using an app (scale 1-100). We also measured the "nocebo" ratio: the ratio of symptoms induced by taking statin that was also induced by taking placebo.
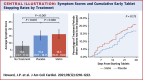
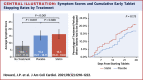
Results: A total of 60 participants were randomized and 49 completed the 12-month protocol. Mean symptom score was 8.0 (95% CI: 4.7-11.3) in no-tablet months. It was higher in statin months (16.3; 95% CI: 13.0-19.6; P < 0.001), but also in placebo months (15.4; 95% CI: 12.1-18.7; P < 0.001), with no difference between the 2 (P = 0.388). The corresponding nocebo ratio was 0.90. In the individual-patient daily data, neither symptom intensity on starting (OR: 1.02; 95% CI: 0.98-1.06; P = 0.28) nor extent of symptom relief on stopping (OR: 1.01; 95% CI: 0.98-1.05; P = 0.48) distinguished between statin and placebo. Stopping was no more frequent for statin than placebo (P = 0.173), and subsequent symptom relief was similar between statin and placebo. At 6 months after the trial, 30 of 60 (50%) participants were back taking statins.
Conclusions: The majority of symptoms caused by statin tablets were nocebo. Clinicians should not interpret symptom intensity or timing of symptom onset or offset (on starting or stopping statin tablets) as indicating pharmacological causation, because the pattern is identical for placebo. (Self-Assessment Method for Statin Side-effects Or Nocebo [SAMSON]; NCT02668016).
Keywords: crossover trial; drug intolerance; nocebo; side effects; statins.
Copyright © 2021 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Funding Support and Author Disclosures This study was funded by the British Heart Foundation (PG/15/7/31235), which had no role in study design, data collection, data analysis, data interpretation, or writing of the report. This study was supported by the National Institute for Health Research Imperial Biomedical Research Centre (BRC) and the Imperial Clinical Trials Unit. The views expressed are those of the author and not necessarily those of the National Institute for Health Research or the Department of Health and Social Care. Dr Howard is supported by the Wellcome Trust, grant number 212183/Z/18/Z. Dr Nowbar is supported by the National Institute for Health Research Academy. Dr Rajkumar is supported by the Medical Research Council, grant number MR/S021108/1. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Figures

Comment in
-
That Myalgia of Yours Is Not From Statin Intolerance.J Am Coll Cardiol. 2021 Sep 21;78(12):1223-1226. doi: 10.1016/j.jacc.2021.07.025. J Am Coll Cardiol. 2021. PMID: 34531022 No abstract available.
References
-
- Toth P., Granowitz C., Hull M., Philip S. Long-term statin persistence is poor among high-risk patients with baseline peripheral artery disease: a real-world administrative claims analysis of the Optum Research Database. J Am Coll Cardiol. 2019;73(9 Suppl 1):1744. doi: 10.1016/s0735-1097(19)32350-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

