'NOPAIN-ROP' trial: Intravenous fentanyl and intravenous ketamine for pain relief during laser photocoagulation for retinopathy of prematurity (ROP) in preterm infants: A randomised trial
- PMID: 34531205
- PMCID: PMC8449965
- DOI: 10.1136/bmjopen-2020-046235
'NOPAIN-ROP' trial: Intravenous fentanyl and intravenous ketamine for pain relief during laser photocoagulation for retinopathy of prematurity (ROP) in preterm infants: A randomised trial
Abstract
Objectives: To investigate if intravenous fentanyl or intravenous ketamine can provide adequate analgesia in preterm infants undergoing laser photocoagulation for retinopathy of prematurity (ROP).
Design: Open-label randomised trial.
Setting: Tertiary care institution.
Participants: Preterm infants who underwent laser photocoagulation for ROP.
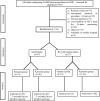
Interventions: Infants were randomised to receive fentanyl as intravenous bolus dose of 2 µg/kg, followed by an intravenous infusion of 1 µg/kg/hour increased to a maximum of 3 µg/kg/hour or intravenous ketamine as bolus dose of 0.5 mg/kg, followed by further intermittent intravenous bolus doses of 0.5 mg/kg to a maximum of 2 mg/kg in the initial phase and intravenous fentanyl (bolus of 2 µg/kg followed by infusion of 2 µg/kg/hour to a maximum of 5 µg/kg/hour) or intravenous ketamine (bolus dose of 1 mg/kg followed by intermittent bolus doses of 0.5 mg/kg to a maximum of 4 mg/kg) in the revised regimen phase.
Main outcome measures: Proportion of infants with adequate analgesia defined as the presence of both: (1) all the Premature Infant Pain Profile-Revised scores measured every 15 min less than seven and (2) proportion of the procedure time the infant spent crying less than 5%.Secondary outcomes included apnoea, cardiorespiratory or haemodynamic instability, feed intolerance and urinary retention requiring catheterisation during and within 24 hours following the procedure.
Results: A total of 97 infants were randomised (fentanyl=51, ketamine=46). The proportions of infants with adequate analgesia were 16.3% (95% CI 8.5% to 29%) with fentanyl and 4.5% (95% CI 1.3% to 15.1%) with ketamine. Ten infants (19.6%) in the fentanyl group and seven infants (15.2%) in the ketamine group had one or more side effects. In view of inadequate analgesia with both the regimens, the study steering committee recommended using a higher dose of intravenous fentanyl and intravenous ketamine. Consequently, we enrolled 27 infants (fentanyl=13, ketamine=14). With revised regimens, the proportions of infants with adequate analgesia were higher: 23.1% (95% CI 8.2% to 50.2%) with fentanyl and 7.1% (95% CI 1.3% to 31.5%) with ketamine. However, higher proportions of infants developed apnoea (n=4; 30.7%), need for supplemental oxygen (n=5, 38.4%) and change in cardiorespiratory scores (n=7; 53.8%) with fentanyl but none with ketamine.
Conclusions: Fentanyl-based and ketamine-based drug regimens provided adequate analgesia only in a minority of infants undergoing laser photocoagulation for ROP. More research is needed to find safe and effective regimens that can be employed in resource constrained settings.
Trial registration number: CTRI/2018/03/012878.
Keywords: intensive & critical care; neonatal intensive & critical care; neonatology; paediatrics; pain management.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Sivanandan S, Chandra P, Deorari AK, et al. . Retinopathy of prematurity: AIIMS, new Delhi experience. Indian Pediatr 2016;53 Suppl 2:S123–8. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources