Intratumoral combination therapy with poly(I:C) and resiquimod synergistically triggers tumor-associated macrophages for effective systemic antitumoral immunity
- PMID: 34531246
- PMCID: PMC8449972
- DOI: 10.1136/jitc-2021-002408
Intratumoral combination therapy with poly(I:C) and resiquimod synergistically triggers tumor-associated macrophages for effective systemic antitumoral immunity
Abstract
Background: Tumor-associated macrophages (TAMs) play a key immunosuppressive role that limits the ability of the immune system to fight cancer and hinder the antitumoral efficacy of most treatments currently applied in the clinic. Previous studies have evaluated the antitumoral immune response triggered by (TLR) agonists, such as poly(I:C), imiquimod (R837) or resiquimod (R848) as monotherapies; however, their combination for the treatment of cancer has not been explored. This study investigates the antitumoral efficacy and the macrophage reprogramming triggered by poly(I:C) combined with R848 or with R837, versus single treatments.
Methods: TLR agonist treatments were evaluated in vitro for toxicity and immunostimulatory activity by Alamar Blue, ELISA and flow cytometry using primary human and murine M-CSF-differentiated macrophages. Cytotoxic activity of TLR-treated macrophages toward cancer cells was evaluated with an in vitro functional assay by flow cytometry. For in vivo experiments, the CMT167 lung cancer model and the MN/MCA1 fibrosarcoma model metastasizing to lungs were used; tumor-infiltrating leukocytes were evaluated by flow cytometry, RT-qPCR, multispectral immunophenotyping, quantitative proteomic experiments, and protein-protein interaction analysis.
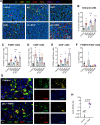
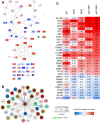
Results: Results demonstrated the higher efficacy of poly(I:C) combined with R848 versus single treatments or combined with R837 to polarize macrophages toward M1-like antitumor effectors in vitro. In vivo, the intratumoral synergistic combination of poly(I:C)+R848 significantly prevented tumor growth and metastasis in lung cancer and fibrosarcoma immunocompetent murine models. Regressing tumors showed increased infiltration of macrophages with a higher M1:M2 ratio, recruitment of CD4+ and CD8+ T cells, accompanied by a reduction of immunosuppressive CD206+ TAMs and FOXP3+/CD4+ T cells. The depletion of both CD4+ and CD8+ T cells resulted in complete loss of treatment efficacy. Treated mice acquired systemic antitumoral response and resistance to tumor rechallenge mediated by boosted macrophage cytotoxic activity and T-cell proliferation. Proteomic experiments validate the superior activation of innate immunity by poly(I:C)+R848 combination versus single treatments or poly(I:C)+R837, and protein-protein-interaction network analysis reveal the key activation of the STAT1 pathway.
Discussion: These findings demonstrate the antitumor immune responses mediated by macrophage activation on local administration of poly(I:C)+R848 combination and support the intratumoral application of this therapy to patients with solid tumors in the clinic.
Keywords: immunity; immunotherapy; innate; lung neoplasms; macrophages.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
