Systematic preclinical evaluation of CD33-directed chimeric antigen receptor T cell immunotherapy for acute myeloid leukemia defines optimized construct design
- PMID: 34531250
- PMCID: PMC8449984
- DOI: 10.1136/jitc-2021-003149
Systematic preclinical evaluation of CD33-directed chimeric antigen receptor T cell immunotherapy for acute myeloid leukemia defines optimized construct design
Erratum in
-
Correction: Systematic preclinical evaluation of CD33-directed chimeric antigen receptor T cell immunotherapy for acute myeloid leukemia defines optimized construct design.J Immunother Cancer. 2021 Oct;9(10):e003149corr1. doi: 10.1136/jitc-2021-003149corr1. J Immunother Cancer. 2021. PMID: 34615707 Free PMC article. No abstract available.
Abstract
Background: Successful development of chimeric antigen receptor (CAR) T cell immunotherapy for children and adults with relapsed/refractory acute myeloid leukemia (AML) is highly desired given their poor clinical prognosis and frequent inability to achieve cure with conventional chemotherapy. Initial experiences with CD19 CAR T cell immunotherapy for patients with B-cell malignancies highlighted the critical impact of intracellular costimulatory domain selection (CD28 vs 4-1BB (CD137)) on CAR T cell expansion and in vivo persistence that may impact clinical outcomes. However, the impact of costimulatory domains on the efficacy of myeloid antigen-directed CAR T cell immunotherapy remains unknown.
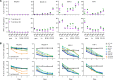
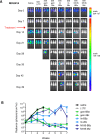
Methods: In this preclinical study, we developed six CAR constructs targeting CD33, a highly expressed and validated AML target, comprised of one of three single-chain variable fragments with CD3ζ and either CD28 or 4-1BB costimulatory domains. We systematically compared the preclinical in vitro and in vivo efficacy of T cells lentivirally transduced with CD33 CAR constructs (CD33CARTs) against human AML.
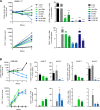
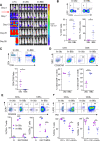
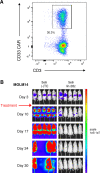
Results: We observed potent in vitro cytokine production and cytotoxicity of CD33CARTs incubated with human CD33+ AML cell lines, as well as robust in vivo antileukemia activity in cell line and childhood AML patient-derived xenograft (PDX) models. Gemtuzumab-based CD33CARTs were unexpectedly toxic in vivo in animal models despite observed in vitro anti-leukemia activity. CD28-based CD33CARTs consistently induced more robust inhibition of leukemia proliferation in AML cell line and PDX models than did 4-1BB-based CD33CARTs. A 'best-in-class' lintuzumab-CD28/CD3ζ CAR construct was thus selected for clinical translation.
Conclusions: CD33 is a critical antigen for potential immunotherapeutic targeting in patients with AML. Based on this rigorous preclinical evaluation, our validated clinical grade lintuzumab-CD28/CD3ζ CD33CART immunotherapy is now under evaluation in a first-in-child/first-in-human phase 1 clinical trial for children and adolescents/young adults with relapsed/refractory AML.
Trial registration number: clinicaltrials.gov; NCT03971799.
Keywords: adoptive; chimeric antigen; hematologic neoplasms; immunotherapy; pediatrics; receptors; translational medical research.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: HQ and TJF have filed United States patent number WO2019178382A1 ‘Anti-CD33 Chimeric Antigen Receptors and Their Uses.’ SKT is a scientific advisory board member for Aleta Biotherapeutics and Kura Oncology and has received research funding from Incyte Corporation and Gilead Sciences for unrelated studies. TJF has received research funding from Lentigen and Elevate Bio for unrelated studies and is a current employee of Sana Biotechnology. The remaining authors declare no relevant conflicts of interest.
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
