Spontaneous ARIA-like Events in Cerebral Amyloid Angiopathy-Related Inflammation: A Multicenter Prospective Longitudinal Cohort Study
- PMID: 34531298
- PMCID: PMC8610623
- DOI: 10.1212/WNL.0000000000012778
Spontaneous ARIA-like Events in Cerebral Amyloid Angiopathy-Related Inflammation: A Multicenter Prospective Longitudinal Cohort Study
Abstract
Background and objectives: The goal of this work was to investigate the natural history and outcomes after treatment for spontaneous amyloid-related imaging abnormalities (ARIA)-like in cerebral amyloid angiopathy-related inflammation (CAA-ri).
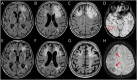
Methods: This was a multicenter, hospital-based, longitudinal, prospective observational study of inpatients meeting CAA-ri diagnostic criteria recruited through the Inflammatory Cerebral Amyloid Angiopathy and Alzheimer's Disease βiomarkers International Network from January 2013 to March 2017. A protocol for systematic data collection at first-ever presentation and at subsequent in-person visits, including T1-weighted, gradient recalled echo-T2*, fluid-suppressed T2-weighted (fluid-attenuated inversion recovery), and T1 postgadolinium contrast-enhanced images acquired on 1.5T MRI, was used at the 3-, 6-, 12-, and 24-month follow-up. Centralized reads of MRIs were performed by investigators blinded to clinical, therapeutic, and time-point information. Main outcomes were survival, clinical and radiologic recovery, intracerebral hemorrhage (ICH), and recurrence of CAA-ri.
Results: The study enrolled 113 participants (10.6% definite, 71.7% probable, and 17.7% possible CAA-ri). Their mean age was 72.9 years; 43.4% were female; 37.1% were APOEε4 carriers; 36.3% had a history of Alzheimer disease; and 33.6% had a history of ICH. A history of ICH and the occurrence of new ICH at follow-up were more common in patients with cortical superficial siderosis at baseline (52.6% vs 14.3%, p < 0.0001 and 19.3% vs 3.6%, p < 0.009, respectively). After the first-ever presentation of CAA-ri, 70.3% (95% confidence interval [CI] 61.6%-78.5%) and 84.1% (95% CI 76.2%-90.6%) clinically recovered within 3 and 12 months, followed by radiologic recovery in 45.1% (95% CI 36.4%-54.8%) and 77.4% (95% CI 67.7%-85.9%), respectively. After clinicoradiologic resolution of the first-ever episode, 38.3% (95% CI 22.9%-59.2%) had at least 1 recurrence within the following 24 months. Recurrence was more likely if IV high-dose corticosteroid pulse therapy was suddenly stopped compared to slow oral tapering off (hazard ratio 4.68, 95% CI 1.57-13.93; p = 0.006).
Discussion: These results from the largest longitudinal cohort registry of patients with CAA-ri support the transient and potentially relapsing inflammatory nature of the clinical-radiologic acute manifestations of the disease and the effectiveness of slow oral tapering off after IV corticosteroid pulse therapy in preventing recurrences. Our results highlight the importance of differential diagnosis for spontaneous ARIA-like events in β-amyloid-driven diseases, including treatment-related ARIA in patients with Alzheimer disease exposed to immunotherapy drugs.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures



Comment in
-
Author Response: Spontaneous ARIA-like Events in Cerebral Amyloid Angiopathy-Related Inflammation: A Multicenter Prospective Longitudinal Cohort Study.Neurology. 2022 Oct 18;99(16):729. doi: 10.1212/WNL.0000000000201353. Neurology. 2022. PMID: 36253125 No abstract available.
-
Reader Response: Spontaneous ARIA-like Events in Cerebral Amyloid Angiopathy-Related Inflammation: A Multicenter Prospective Longitudinal Cohort Study.Neurology. 2022 Oct 18;99(16):728-729. doi: 10.1212/WNL.0000000000201359. Neurology. 2022. PMID: 36253126 No abstract available.
References
-
- Piazza F, Greenberg SM, Savoiardo M, et al. . Anti-amyloid beta autoantibodies in cerebral amyloid angiopathy-related inflammation: implications for amyloid-modifying therapies. Ann Neurol. 2013;73(4):449-458. - PubMed
-
- Auriel E, Charidimou A, Gurol ME, et al. . Validation of clinicoradiological criteria for the diagnosis of cerebral amyloid angiopathy-related inflammation. JAMA Neurol. 2016;73(2):197-202. - PubMed
-
- Castro Caldas A, Silva C, Albuquerque L, Pimentel J, Silva V, Ferro JM. Cerebral amyloid angiopathy associated with inflammation: report of 3 cases and systematic review. J Stroke Cerebrovasc Dis. 2015;24(9):2039-2048. - PubMed
-
- Corovic A, Kelly S, Markus HS. Cerebral amyloid angiopathy associated with inflammation: a systematic review of clinical and imaging features and outcome. Int J Stroke. 2018;13(3):257-267. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
