Yield of clinically reportable genetic variants in unselected cerebral palsy by whole genome sequencing
- PMID: 34531397
- PMCID: PMC8445947
- DOI: 10.1038/s41525-021-00238-0
Yield of clinically reportable genetic variants in unselected cerebral palsy by whole genome sequencing
Abstract
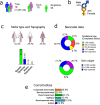
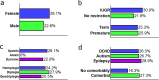
Cerebral palsy (CP) is the most common cause of childhood physical disability, with incidence between 1/500 and 1/700 births in the developed world. Despite increasing evidence for a major contribution of genetics to CP aetiology, genetic testing is currently not performed systematically. We assessed the diagnostic rate of genome sequencing (GS) in a clinically unselected cohort of 150 singleton CP patients, with CP confirmed at >4 years of age. Clinical grade GS was performed on the proband and variants were filtered, and classified according to American College of Medical Genetics and Genomics-Association for Molecular Pathology (ACMG-AMP) guidelines. Variants classified as pathogenic or likely pathogenic (P/LP) were further assessed for their contribution to CP. In total, 24.7% of individuals carried a P/LP variant(s) causing or increasing risk of CP, with 4.7% resolved by copy number variant analysis and 20% carrying single nucleotide or indel variants. A further 34.7% carried one or more rare, high impact variants of uncertain significance (VUS) in variation intolerant genes. Variants were identified in a heterogeneous group of genes, including genes associated with hereditary spastic paraplegia, clotting and thrombophilic disorders, small vessel disease, and other neurodevelopmental disorders. Approximately 1/2 of individuals were classified as likely to benefit from changed clinical management as a result of genetic findings. In addition, no significant association between genetic findings and clinical factors was detectable in this cohort, suggesting that systematic sequencing of CP will be required to avoid missed diagnoses.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
References
Grants and funding
- 1099163/Department of Health | National Health and Medical Research Council (NHMRC)
- 1041920/Department of Health | National Health and Medical Research Council (NHMRC)
- 1099163/Department of Health | National Health and Medical Research Council (NHMRC)
- 1099163/Department of Health | National Health and Medical Research Council (NHMRC)
- 1099163/Department of Health | National Health and Medical Research Council (NHMRC)
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

