Activation of anionic redox in d0 transition metal chalcogenides by anion doping
- PMID: 34531403
- PMCID: PMC8445930
- DOI: 10.1038/s41467-021-25760-8
Activation of anionic redox in d0 transition metal chalcogenides by anion doping
Abstract
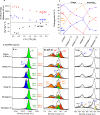
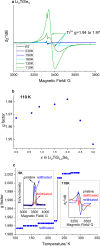
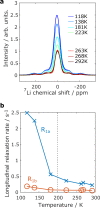
Expanding the chemical space for designing novel anionic redox materials from oxides to sulfides has enabled to better apprehend fundamental aspects dealing with cationic-anionic relative band positioning. Pursuing with chalcogenides, but deviating from cationic substitution, we here present another twist to our band positioning strategy that relies on mixed ligands with the synthesis of the Li2TiS3-xSex solid solution series. Through the series the electrochemical activity displays a bell shape variation that peaks at 260 mAh/g for the composition x = 0.6 with barely no capacity for the x = 0 and x = 3 end members. We show that this capacity results from cumulated anionic (Se2-/Sen-) and (S2-/Sn-) and cationic Ti3+/Ti4+ redox processes and provide evidence for a metal-ligand charge transfer by temperature-driven electron localization. Moreover, DFT calculations reveal that an anionic redox process cannot take place without the dynamic involvement of the transition metal electronic states. These insights can guide the rational synthesis of other Li-rich chalcogenides that are of interest for the development of solid-state batteries.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- Assat G, Tarascon J-M. Fundamental understanding and practical challenges of anionic redox activity in Li-ion batteries. Nat. Energy. 2018;3:373–386. doi: 10.1038/s41560-018-0097-0. - DOI
-
- Jiang J, Eberman KW, Krause LJ, Dahn JR. Structure, electrochemical properties, and thermal stability studies of cathode materials in the xLi[Mn1/2Ni1/2]O2⋅yLiCoO2⋅zLi[Li1/3Mn2/3]O2 pseudoternary system (x+y+z=1) J. Electrochem. Soc. 2005;152:A1879. doi: 10.1149/1.1995690. - DOI
-
- Thackeray MM, et al. Li2MnO3-stabilized LiMO2 (M = Mn, Ni, Co) electrodes for lithium-ion batteries. J. Mater. Chem. 2007;17:3112–3125. doi: 10.1039/b702425h. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources

